Thu, Jan 29, 2026
Volume 15, Issue 4 (Autumn 2025)
PTJ 2025, 15(4): 307-322 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sakinepoor A, Degens H, Letafatkar A, Darabseh M Z, Moradi F, Mazidi M. The Effect of Exercises on Flexion Relaxation in Adolescents With Forward Head Posture and Rounded Shoulders. PTJ 2025; 15 (4) :307-322
URL: http://ptj.uswr.ac.ir/article-1-678-en.html
URL: http://ptj.uswr.ac.ir/article-1-678-en.html
Ainollah Sakinepoor *1 

 , Hans Degens2
, Hans Degens2 

 , Amir Letafatkar3
, Amir Letafatkar3 

 , Mohammad Z. Darabseh4
, Mohammad Z. Darabseh4 

 , Fariba Moradi5
, Fariba Moradi5 

 , Maryam Mazidi6
, Maryam Mazidi6 




 , Hans Degens2
, Hans Degens2 

 , Amir Letafatkar3
, Amir Letafatkar3 

 , Mohammad Z. Darabseh4
, Mohammad Z. Darabseh4 

 , Fariba Moradi5
, Fariba Moradi5 

 , Maryam Mazidi6
, Maryam Mazidi6 


1- Department of Physical Education, Farhangian University, Tehran, Iran.
2- Department of Life Sciences, Manchester Metropolitan University, Manchester, United Kingdom. & Lithuanian Sports University, Kaunas, Lithuania.
3- Department of Biomechanics and Sport Injuries, Faculty of Physical Education, Kharazmi University, Tehran, Iran.
4- Department of Physiotherapy, School of Rehabilitation Sciences, The University of Jordan, Amman, Jordan.
5- Department of Physical Education, Faculty of Sports Sciences, Arak University, Arak, Iran.
6- Department of Physical Education, Faculty of Humanities, Hormozgan University, Bandar Abbas, Iran.
2- Department of Life Sciences, Manchester Metropolitan University, Manchester, United Kingdom. & Lithuanian Sports University, Kaunas, Lithuania.
3- Department of Biomechanics and Sport Injuries, Faculty of Physical Education, Kharazmi University, Tehran, Iran.
4- Department of Physiotherapy, School of Rehabilitation Sciences, The University of Jordan, Amman, Jordan.
5- Department of Physical Education, Faculty of Sports Sciences, Arak University, Arak, Iran.
6- Department of Physical Education, Faculty of Humanities, Hormozgan University, Bandar Abbas, Iran.
Keywords: Exercise therapy, Posture, Postural balance forward head, Forward shoulder, Electromyography
Full-Text [PDF 1026 kb]
(4237 Downloads)
| Abstract (HTML) (1582 Views)
Full-Text: (533 Views)
Introduction
Today’s teenagers are highly aware of the media and frequently use advanced technologies, such as smartphones [1]. The 2021 report on digital news users in Spain, released by the University of Navarra and University of Oxford, reveals that the cell phone is the predominant device utilized by Internet consumers for information retrieval. Specifically, 90% of users regularly engage with their cell phones for various purposes, and 78% utilize them for news consumption. This represents a five-percentage-point increase compared to 2020 and an 11-point rise from 2019, when the figure stood at 67%. Additionally, insights from the most recent annual report of the national observatory of telecommunications and the information society further support these findings [2]. This constant engagement with devices makes adolescents vulnerable to negative impacts, particularly regarding posture. Extended use of smartphones, laptops, and other screens can lead to common postural issues, such as forward head angle (FHA) and rounded shoulder angle (RSA) [3]. The occurrence of forward head posture (FHP) and rounded shoulder posture (RSP) among a cohort of healthy individuals aged 20-50 years was documented, revealing that 66% exhibited FHP, 73% displayed right RSP, and 66% presented left RSP [4]. In adolescents, the prevalence of common postural abnormalities indicated that the most frequently observed issue was uneven shoulder height (36%), followed by FHP (25%) [4]. Additionally, individuals with FHP demonstrate increased extension of the atlantooccipital joint and upper cervical spine, which is linked to flexion in the lower cervical and upper thoracic spine [5]. RSP is defined by a protruded acromion process of the shoulder joint about the gravitational line, resulting in a stooped posture characterized by elevation, protraction, and downward rotation of the scapula. Furthermore, this condition leads to an increased angle between the lower cervical vertebrae and the upper spine [6].
During computer work, the cervical erector spinae (CES) muscle plays a crucial role in effective activation and support for the task at hand. According to Yoo et al. [6], the fatigue experienced by the CES muscle as a result of tasks involving visual display terminals can be measured using the flexion relaxation phenomenon (FRP). This phenomenon is characterized by a lack of electrical activity in the erector spinae (ES) muscles when the trunk is fully flexed [7, 8]. The FRP occurs because the load shifts from the active muscles (ES) to the passive structures of the spine, such as ligaments, capsules, and intervertebral discs [9, 10]. The FRP observed in the cervical spine mirrored that of the ES muscle. During neck flexion, the cervical extensors gradually increase their activation to manage the increasing load from the head [11]. Once the head is completely flexed, the responsibility of supporting this load shifts from the active muscles to the passive structures, leading to a decrease or cessation of myoelectric activity in the muscles [12]. This interplay between the active and passive components is essential for maintaining the mechanical stability of the spine and its neural system [13].
However, research indicates that these postures, resulting from prolonged neck flexion, place static strain on the musculoskeletal system and increase compressive stress on the cervical spine. Over time, this can lead to detrimental changes in spinal soft tissues and negatively affect neck muscle function [4-6]. The interplay of creep and extended static loading can lead to increased looseness in the lower back, thereby compromising stability of the spinal column [7, 8]. Attaining spinal stability depends on well-coordinated collaboration between the active and passive elements of the neuromusculoskeletal system. In the neck region of the spine, passive stability is provided by the viscoelastic characteristics of the spinal structures [9], whereas active stability arises from both intentional and reflexive muscle activation [9]. Multiple studies examining the lumbar spine have suggested that prolonged trunk flexion leads to a reduction in passive support [10] and that active stiffness is crucial for maintaining spinal stability when passive support is lacking [9].
Researchers investigating the interplay between passive and active stabilizers often utilize FRP [11]. The FRR is the proportion of the highest activation during the re-expansion stage to the mean activation observed during the maximum bending position stage (the quiet period) [12].
This phenomenon explains how muscles and viscoelastic structures, such as ligaments, disks, capsules, and fascia, collaborate to distribute loads effectively [13]. As neck flexion occurs, cervical extensors progressively enhance their activation to counteract the gravitational pull on the head’s position. When the completely bent position, the stressed viscoelastic structures generate an encumbrance enough to counteract gravity, resulting in decreased activation of the extensor muscles [14]. Some studies have noted the lack or postponement of FRP during full neck flexion, especially among participants experiencing neck pain [15].
Abnormal flexion-relaxation patterns can be improved through exercise interventions [16, 17], which may also help rectify muscle imbalances that lead to movement compensations associated with Letafatkar et al. [16] For example, deep cervical flexor strengthening exercises are advised to mitigate FH and RS and promote an upright posture [18]. Many studies have examined how exercise affects flexion-relaxation patterns in people experiencing low back pain [16, 19], and FH and RS [20]; however, there is a deficiency of substantial information and consensus regarding the influence of therapeutic exercise routines on these patterns. Some therapies have demonstrated no effect on the flexion-relaxation response (FRP) [20-22], whereas other studies have indicated an improvement in the flexion-relaxation pattern following therapeutic exercise [23, 24].
A therapeutic exercise program was created to focus on alterations in posture, center of gravity, and base of support in the three participants. The regimen was divided into three stages: The initial stage focused on slow, controlled movements to alleviate pain, enhance muscle collaboration, and improve proprioception; the secondary stage aimed at building muscular endurance; and the final stage emphasized muscle strengthening. The participants were instructed to perform each exercise for approximately 30-60 s. Furthermore, the program provided instructions on how to realign the spine, scapula, glenohumeral joint, cervical, and stomach during each meeting, highlighting the importance of maintaining these alignments against a wall or bed whenever possible before starting the therapeutic exercises.
The procedure assists in preventing deviation of neck and waist lordosis, as well as roundback, while performing exercises [25]. However, to our knowledge, no study with random assignment to groups (randomized controlled trials [RCT]) has examined the effectiveness of corrective exercise (CE) on the FRP in participants with flexion-related symptomatic postural impairments. Additionally, evidence regarding the impact of CE on FRP is limited and lacks consensus; to date, no studies have utilized CE to enhance flexion-relaxation. Moreover, these exercises require no special equipment or facilities and can be easily performed at home.
The target was to ascertain the benefits of training on FRP and posture in individuals with FHP and RSP. We hypothesized that CE would improve flexion-relaxation and postural misalignments in individuals with FHA and RSA postures after 8 weeks. The control group did not undergo training and engaged in their regular daily activities.
Materials and Methods
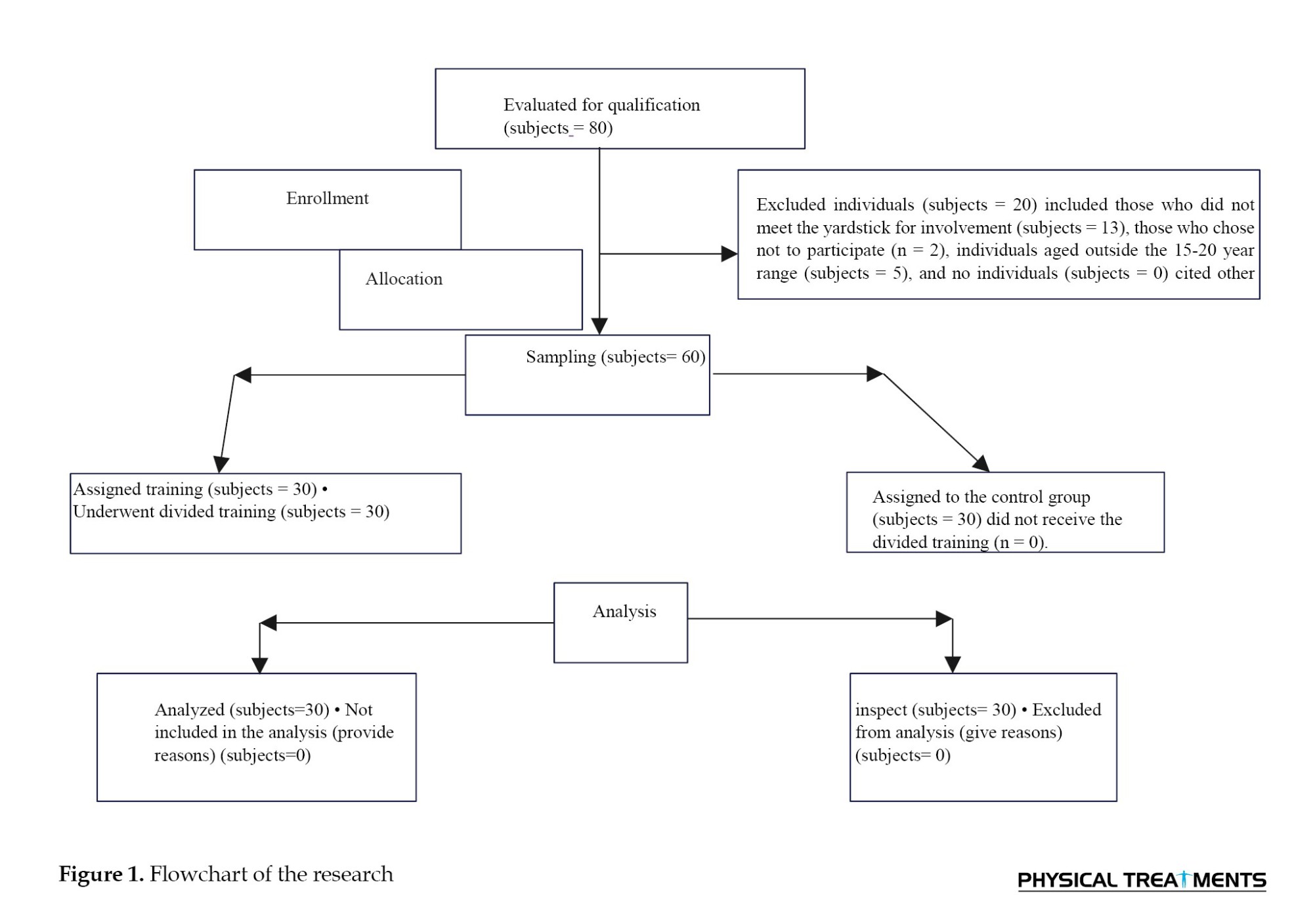
A randomized controlled trial (RCT) was conducted, and ethical clearance was obtained from the Ethics Committee of Hormozgan University of Medical Sciences. Initially, 80 participants were recruited from a university physical therapy clinic that serves clients from the surrounding community. The yardstick for involvement is among 15- to 20-year-olds, having a body mass index (BMI) of 20-25 kg/m², a forward shoulder angle (FSA) exceeding 52°, and an FHA greater than 46°, with these angles measured through photogrammetry (Figure 1). Subjects were omitted if they had a chronicle of cervical spine or back surgery, exhibited neurological symptoms, suffered from atrophic arthritis impacting the neck or back, were currently taking muscle relaxants, engaged in regular physical activity each week, or were professional athletes [26], non-completion of the training program according to the research, lack of willingness of participants to continue participating in the research, non-participation of the participants in two consecutive training sessions, injury during the execution of the exercises [27, 28]. Next, applying the yardstick for involvement and elimination, an expert in physical therapy selected 60 participants. The sample size was established based on preliminary analysis utilizing G*Power software, with the FHA score serving as the primary outcome variable. (Figure 1). Assessments were performed at the beginning of the research and again after 8 weeks at the university’s physical therapy clinic. Following the initial assessment, participants were allocated to one of two groups: Group 1 (CE) and Group 2 (Control). Group 1 underwent a supervised intervention for 8 weeks, while the control group carried out their daily activities. Randomization was implemented using a computerized random number generator. The allocation sequence was kept hidden from the researcher responsible for enrolling and evaluating participants using sequentially numbered, opaque, sealed envelopes. Participants were partially blinded, as they did not know the expected diversity among the groups, but were aware of the treatment they were receiving.
The BioPrint system for postural analysis was utilized to assess posture (Biotonix Inc., Montreal, CA). Markers were affixed to specific anatomical points, including the right tragus of the ear, acromion process, and C7 spinous process. The participants were then guided to position themselves 40 cm from a backdrop, perform three forward bends, reach overhead three times, and ultimately stand upright while looking directly ahead in their usual sleeping position. A digital camera (Canon Power Shot 95, USA) was mounted on a 1-meter-high tripod, positioned 3.5 m from the wall. Photographs were captured from the right side of the participants in sagittal sitting posture. Measurements of FHA and FSA were obtained utilize photo processing software (Adobe Photoshop) as follows: FHA was determined from the vertical anterior line connecting the tragus and the C7 label, while FSA was assessed from the upright posterior line associated with the C7 marker and the acromial label. Normative data indicate that an FSA greater than 52° suggests RSA, and an FHA greater than 46° indicates FHA [18].
In a cervical flexion-relaxation experiment, cervical extensor muscle activity was recorded using electromyography (EMG) during full cervical flexion (Figure 2). The participants started in a vertical, normal cervical spine position, flexed to their maximum extent, and then returned to the normal position. While executing this task, the participants sat vertically on a stool, with their hips and knees at 90° and feet resting on the floor shoulder-width apart. Their shoulders were aligned with their trunk at approximately 90° internal rotation, and their forearms were pronated, with hands relaxed on their hips. The cervical flexion-relaxation procedure consisted of five stages, each lasting three seconds: Phase 1 involved maintaining a normal neck position; phase two required uttermost neck flexion; phase 3 was a hold at maximal cervical flexion; stage four was cervical extension back to the neutral position; and stage five was a hold at the common position [29]. To ensure consistency in speed and duration across all phases, the assistant counted in synchrony with a metronome set to one beat per second [30]. The participants were instructed to maintain a steady, vertical trunk posture to avoid bending or tilting, and to focus on a fixed point directly ahead to maintain the initial head position. Before the flexion-relaxation task, participants practiced with the metronome and the rhythm of head motions until they could consistently perform the task. These tasks were performed three times in succession without breaks between sets.
EMG
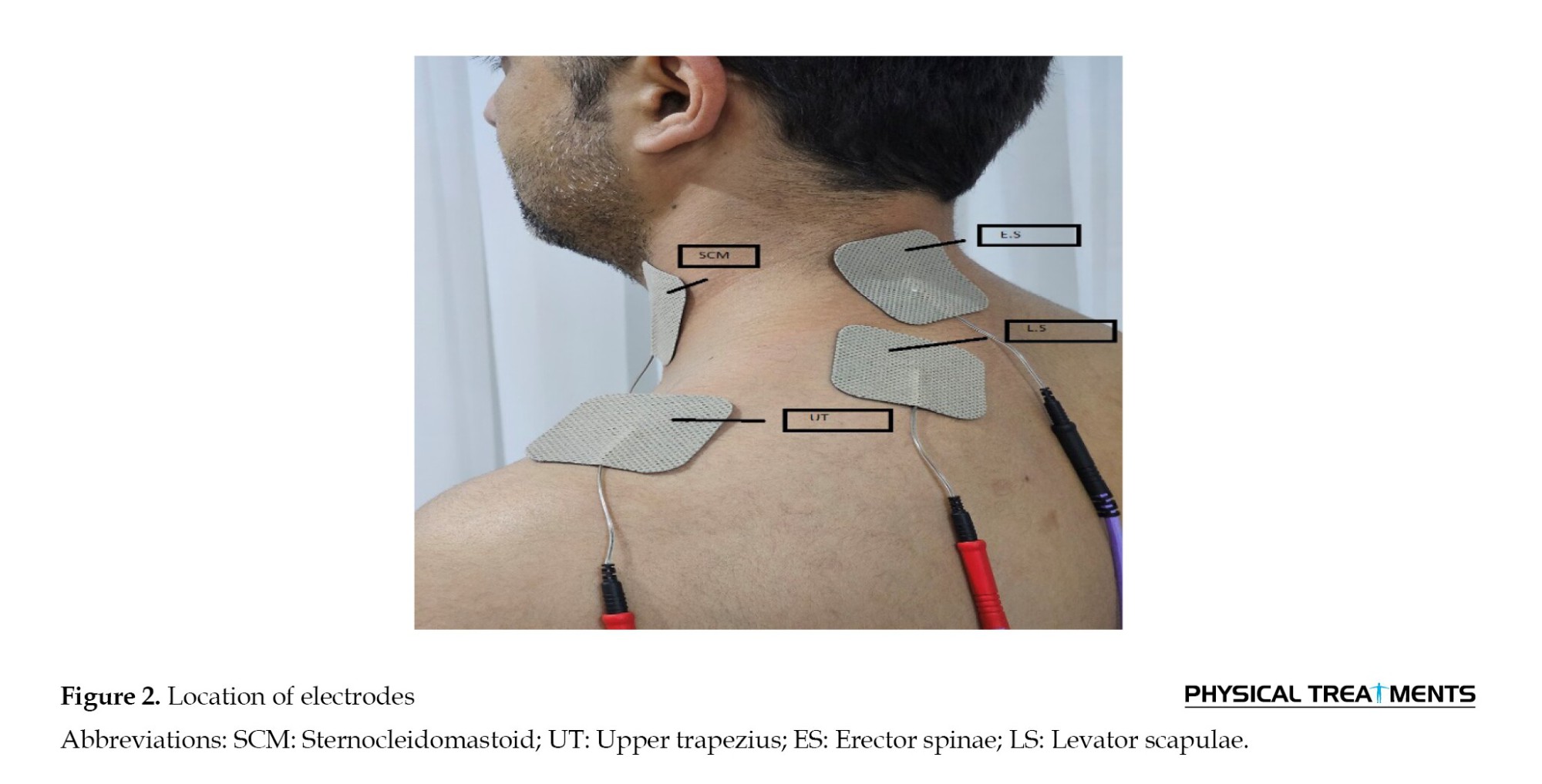
In the electrode placement stage, disposable surface electrodes (models SKINTACT, made in Austria) were used. The center-to-center distance between the electrodes was approximately two and a half centimeters. Initially, the skin was shaved and sanded to decrease skin resistance and improve the quality of the received surface electromyographic (SEMG) signals, and it was cleaned with 70% alcohol. Additionally, the electrodes were placed along the orientation of the muscle fibers by the SENIAM guidelines.
After shaving and polishing the skin at the electrode sites, surface EMG signals were captured from four muscles using a Bagnoli-16 system (FREE EMG 300, BTS Bioengineering, Italy) with a sampling rate of 1000 Hz and a bandwidth of 20–450 Hz. Four pairs of active single-differential dry surface electrodes were evenly positioned on the sternocleidomastoid (SCM), upper trapezius (UT), ES, and levator scapulae (LS) muscles, in line with the established placement protocols (Figure 2). For the UT muscle, the electrode is positioned bilaterally between the spinous process of the C7 spine and the acromion [31]. The SCM electrodes are placed near a point that is thirty percent of the distance from the sternal notch to the mastoid process, straight over the muscle belly of the sternal head [32]. The electrodes for the ES are located 2 cm lateral to the spinous processes of C4 and C5 [32]. In contrast, the electrodes for the LS are positioned betwixt the anterior border of the UT and the posterior border of the SCM [31].
The functional muscle proportion was determined by measuring the greatest muscular activity (one-secondary root mean square [RMS]) of every muscle while in a flexed head position and then dividing this value by the one-second RMS of greatest voluntary contraction for the corresponding muscle.
The entire process of analyzing EMG signals was carried out using MATLAB software. First, EMG data were recorded and stored using a wireless device of brand 16 with a sampling frequency of 1000 Hz. Then, the statistics noise was filtered with a bandwidth of 10 to 450 Hz. The recorded data were analyzed using the RMS method to determine the level of activity. To normalize the data, the activity of every muscle was expressed as a percentage of the maximum RMS during normal activity.
Interventions protocol
The intervention group underwent an 8-week therapeutic exercise routine (Table 1). The exercises were performed twice a week for approximately 20–30 minutes [18]. The intensity of the CE was set to a rating of perceived exertion (RPE) of 11–13 (RPE, Borg’s 6–20 scale), which corresponds to a light-to-somewhat hard training intensity [32]. Most exercises were planned according to the TER principles, with each exercise targeting posture, the focal point of stability and the support foundation. The training regimen included exercises designed to strengthen and stretch the muscles. Exercise advancement was developed in accordance with the findings of an earlier study [33].
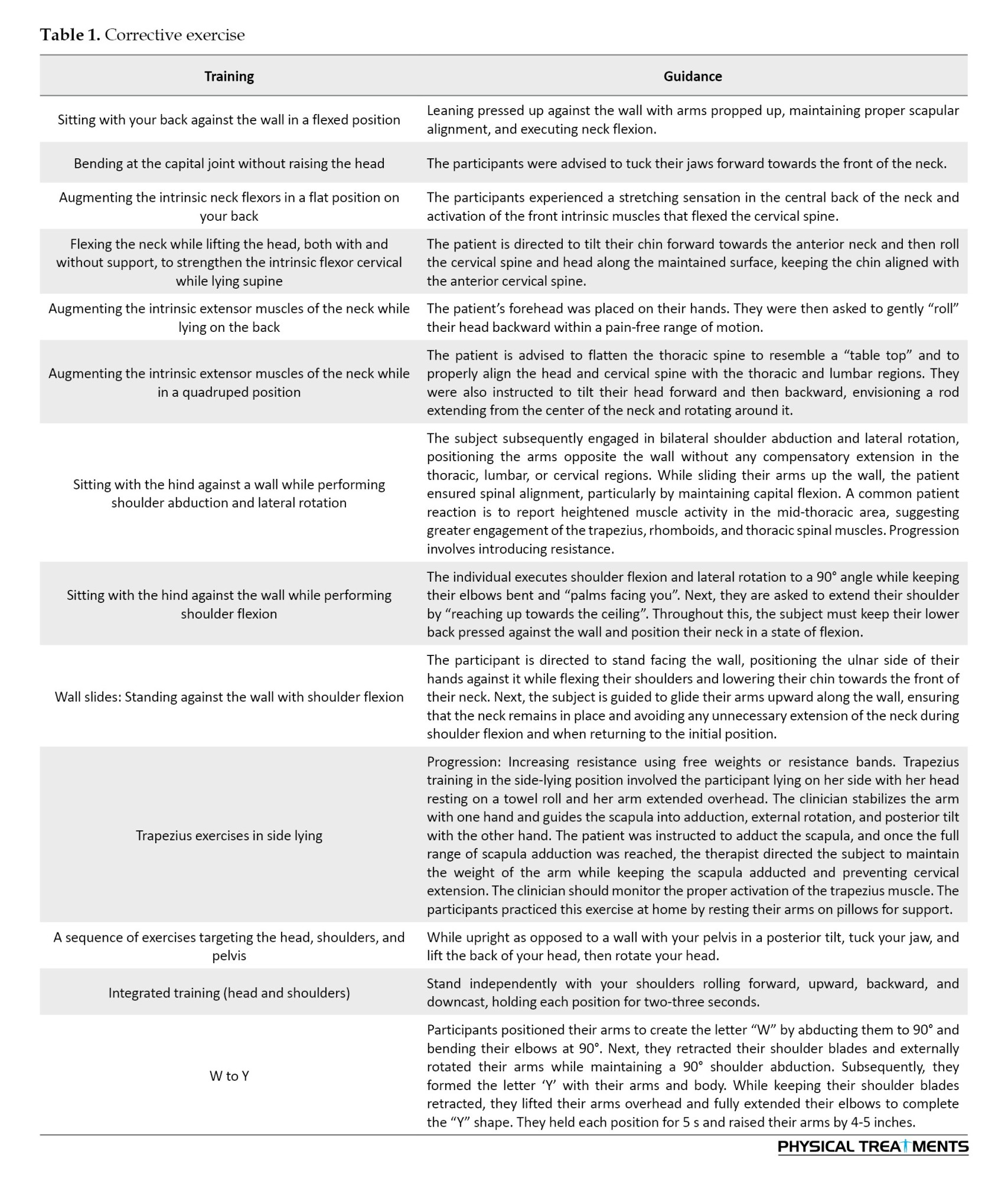
The intervention consisted of three distinct phases. Every initial phase focused on performing slow and controlled training that caused little discomfort, aimed at enhancing muscle coordination and proprioception. The secondary period prioritizes building muscular endurance. The final stage concentrated on muscle reinforcement [33]. Each training session was performed for a duration of 30-60 s [16]. During the second stage, the participants were required to complete three sets of 15 reiterations, with the initial 12 reiterations performed at maximum force, allowing for a 1-minute rest between sets. In the third phase, the participants were encouraged to perform as many reiterations as possible, targeting three sets of 15 reiterations. The control group received advice on how to correct posture. This advice included the following: 1) Reduce neck extension and forward movement of the neck while performing daily tasks. 2) While sitting at the computer, have a supportive chair that will decrease thoracic flexion and help conserve good thoracic posture [16]: 3) Support your forearms either on the desk or an extended tray for a keyboard. The desk or tray should be at the appropriate height, so you do not need to “slouch” for your arms to be supported; 4) Alignment correction when wearing glasses should follow the same sequence that has been demonstrated in the sitting back-to-wall exercises: Start with correction of lumbar, thoracic, and scapular alignment, and then neck and head position; 5) During daily activities, amend your alignment and decrease the stress imposed by adjacent joints before initiating cervical movements [16], and particularly correct the position of your thoracic vertebrae and shoulder girdle, and support your upper limbs; 6) Apply your abdominals to maintain normal lumbar spine alignment and prevent thoracic flexion or “slumping”, particularly when sitting [16].
Statistical analysis
Data were analyzed using the SPSS software, version 21.0 (IBM Corporation, Chicago, IL). To assess the normality of the data, a Kolmogorov-Smirnov test was employed. A 2×2 mixed repeated measures design was implemented to evaluate and compare changes over time, and determine whether these changes varied between the control and TER groups. The significance level was set at <0.05. Effect sizes and 95% confidence intervals (CIs) were calculated to assess clinical significance.
Results
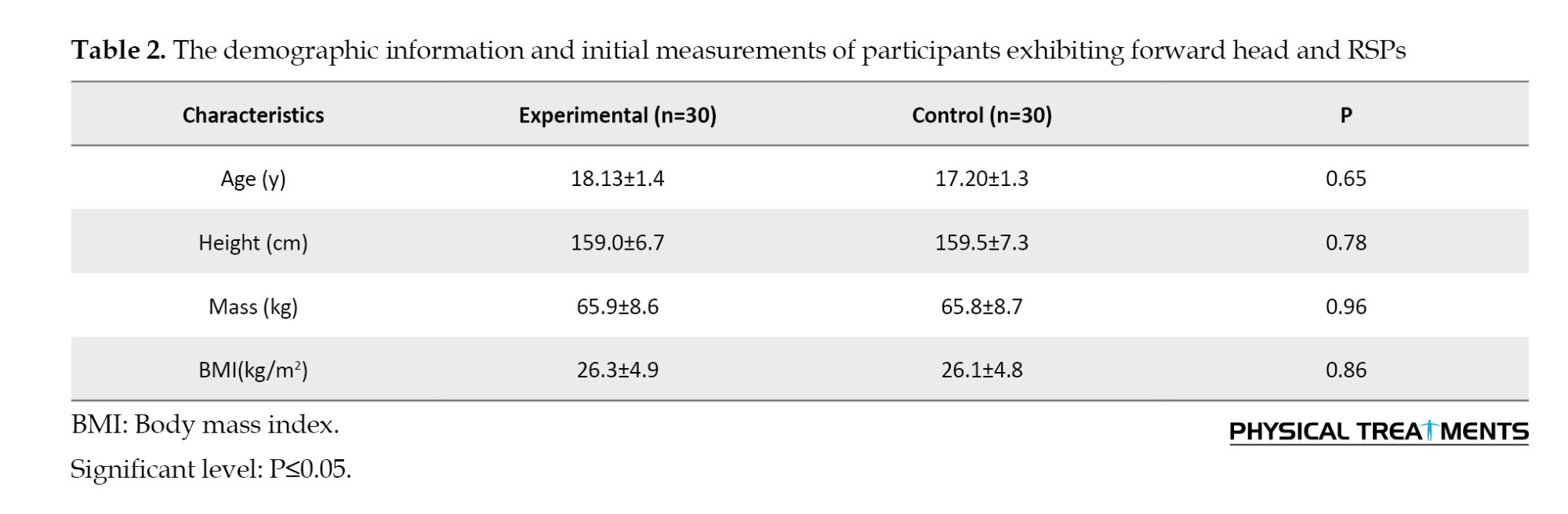
The two groups were similar at the beginning of the study because no significant differences were observed (P>0.05) in their demographic and clinical characteristics (Table 2).
Treatment effects
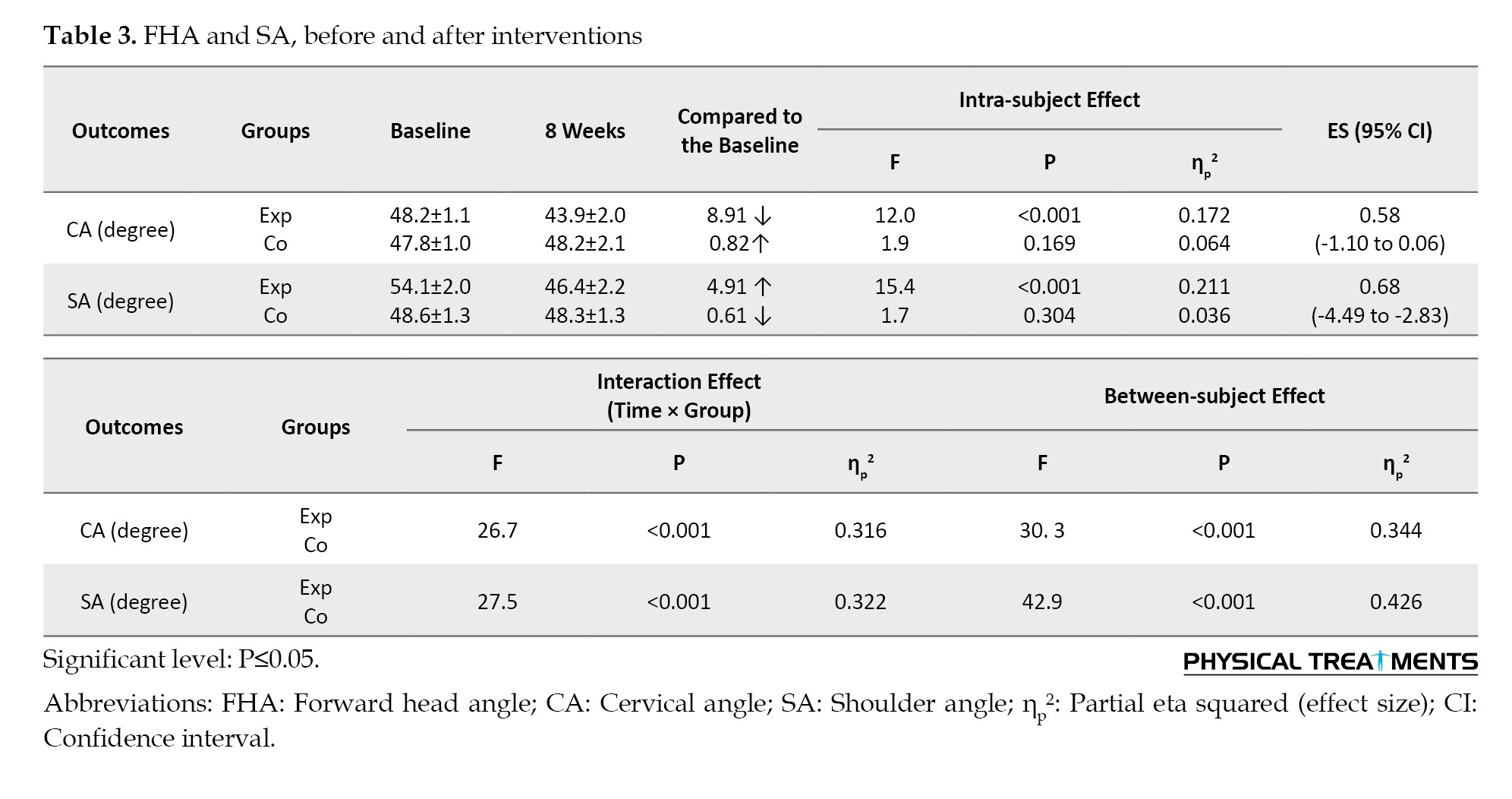
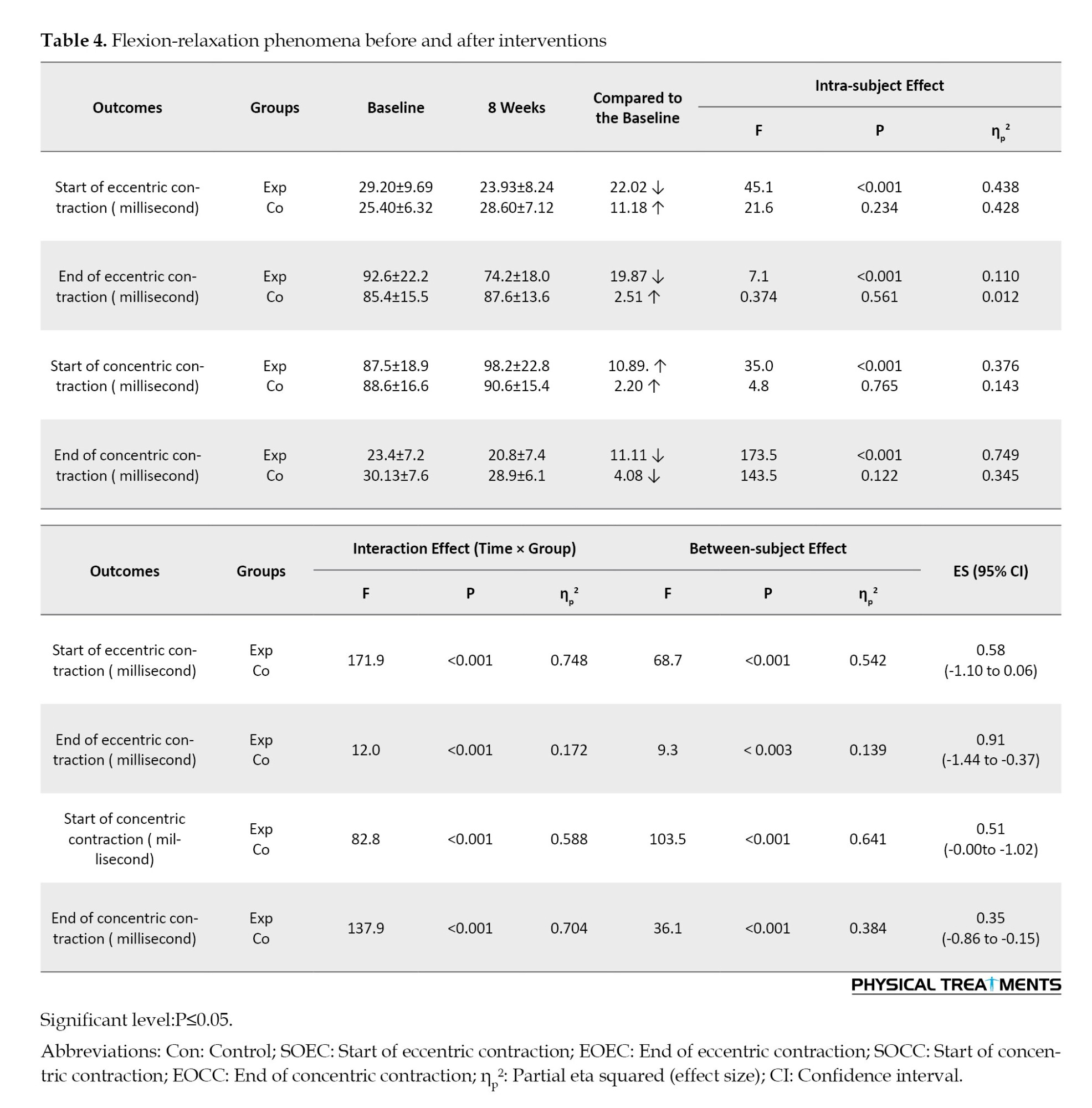
There were main effects of time (P<0.001) and group (P<0.001), as well as a group×time interaction (P<0.001) for FHA, RSA, start and end of eccentric contraction, start and end of concentric contraction, SCM, LS, RMS, UT, and ES. These interactions indicate that the changes over the 8 weeks differed between the control and CE groups.
Only in the CE, but not in the control group, the FHA (P<0.001, ES=0.58, 95% CI, 1.10%, 0.06%) and RSA (P<0.001, ES=0.68, 95% CI, -4.94%, -2.83%) were reduced.
In the FE task, the participants in the CE group observed significantly less RSA (P<0.001, ES=0.68, 95% CI, -4.49%, -2.83%).
In the FE task, participants in the CE group demonstrated a significantly earlier onset of eccentric contraction after the intervention than those in the control group (P<0.001, ES=0.58, 95% CI, -1.10%, 0.06%). Additionally, the participants in the experimental group also demonstrated a significantly earlier cessation of eccentric contraction post-intervention than those in the control group (P=0.000, ES=0.91, 95% CI, -1.44%, -0.37%). Furthermore, the CE group exhibited a significantly delayed onset of concentric contraction after the intervention compared to the control group (P=0.000, ES=0.51, 95% CI, -0.00%, -1.02%). Lastly, the CE group showed a significantly earlier end of concentric contraction post-intervention than to the control group (P=0.001, ES=0.35, 95% CI, -0.86%, -0.15%).
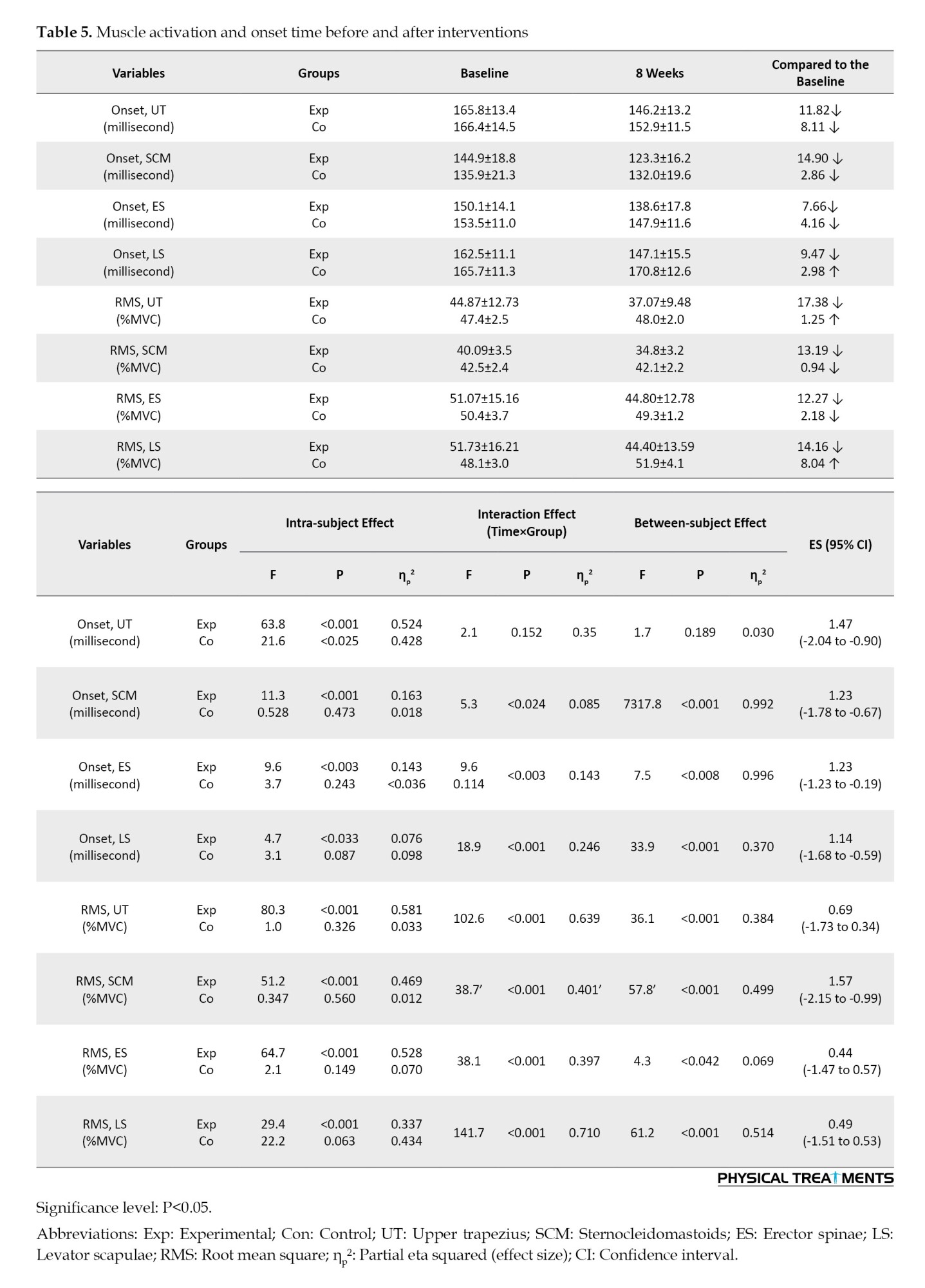
In the FE task, participants in the CE group demonstrated a significantly earlier onset for UT at the post-intervention stage compared to the control group (P<0.001), with an effect size of 1.47 (95% CI, -2.04%, -0.90%). Similarly, for SCM, the TER group also demonstrated a significantly earlier onset post-intervention relative to the control group (P<0.001), with an effect size of 1.23 (95% CI, -1.78%, -0.67%). Additionally, the CE group exhibited a significantly earlier onset for ES (P<0.003), with an effect size of 1.23 (95% CI, -1.23%, -0.19%). For LS, the CE group again showed a significantly earlier onset later-intervention compared to the control group (P=0.000), with an effect size of 1.14 (95% CI, -1.68%, -0.59%). While there was a significant main effect of time (P<0.000), there was no significant group effect (P<0.189) or interaction among group and time for onset, UT (P<0.152).
In the FE task, participants in the CE group demonstrated a significantly earlier RMS for UT at post-intervention compared to the control group (P<0.001), with an effect size (ES) of 0.69 (95% CI, -1.73%, 0.34%). Similarly, the CE group showed a significantly earlier RMS for SCM (P<0.001), with an ES of 1.57 (95% CI, -2.15%, -0.99%). There was a significant main effect of time (P<0.001) and group (P<0.001), along with a notable interaction between group and time on RMS and UT (P<0.001). Additionally, the CE group exhibited a significantly earlier RMS for ES (P<0.001), with an ES of 0.44 (95% CI, -1.47%, 0.57%), and LS (P<0.001), with an ES of 0.49 (95% CI, -1.51%, 0.53%) (Tables 3, 4 and 5).
Discussion
This study aimed to investigate the effects ofCE on FRP and posture in individuals with flexion-related postural dysfunction. The results showed that participants in the CE group showed notable enhancements in both FRP and posture after an eight-week exercise program. The intervention group showed an improvement in the craniovertebral and shoulder angles after training, while the control group did not exhibit any notable changes in these angles. Studies have repeatedly indicated that individuals with forward head and FSA (FHRSA) frequently display irregular flexion-relaxation patterns. The findings of this study confirm that the irregular FRP observed in individuals with FHRS can be significantly improved by applying TER focused on the cervical spine, leading to better FRP outcomes.
Many studies have investigated how stretching programs can enhance range of motion [34, 35]. Certain evaluations suggest that engaging in strength training while the muscle is in an extended position may lead to structural changes [36]. Strength training leads to an enlargement of the muscle’s cross-sectional area by increasing the number of parallel sarcomeres. Additionally, this type of exercise modifies the number of serial sarcomeres, which in turn influences muscle length. The specific length at which muscles are activated during strength training is crucial. Our results are consistent with numerous previous studies [20, 37]. Mak et al. conducted a study on the functional recovery rate (FRR) associated with bending from a seated position in individuals suffering from low back pain (LBP) after undergoing a rehabilitation program. Their findings indicated an improvement in FRR, which was determined by calculating the ratio of RMS values in an upright sitting position to those in a flexed sitting position. A significant rise in the FRR was observed in LBP patients when comparing their status before and after rehabilitation; however, our method indicated a reduction (P<0.05) [18]. One reason for the inconsistency between the results of this study and our research is that our study focused on adolescents, whereas this study focused on adults. Additionally, our research sample included only of boys, while this study included both men and women. Furthermore, the research conducted by Mak et al. measured lumbar muscle flexion relaxation, whereas our study measured neck muscle flexion relaxation. Moreover, the positions used to measure muscle activity differed between the two studies. In our study, the movement involved the neck, while in the study by Mak at al., the assessment was conducted in a sitting position with bending movements and returning from a bent position to an upright sitting position [18].
Furthermore, Neblett et al. investigated variations in FRRs in a cohort of 54 patients with LBP both before and after a back rehabilitation program [38]. The treatment strategy included counseling for stress management and SEMG biofeedback to facilitate relaxation of trunk muscles during flexion. Following treatment, the proportion of patients with chronic low back pain (CLBP) who exhibited normal functional recovery patterns increased significantly, from 30% to 95%. Notably, this study indicated that exercise could effectively normalize abnormal FRP [39]. However, our method indicated a reduction (P<0.05).
One reason for the discrepancies between the results of this study and those of Neblett et al. [38] is that the exercises performed in their study differed from those in our research. Additionally, in the study above, the participants experienced back pain, whereas in our study, the individuals had FHP and forward shoulder posture (FSP). Furthermore, the methods used to measure muscle activity differed between studies.
In addition, Park and Choi examined how stabilization training influences FRP in the erector spinae muscles. Their outcomes suggest that lumbar stabilization training can alleviate FRP asymmetry in these muscles, potentially decreasing the incidence of low back pain in the general population [37]. Our research supports their findings, indicating that following a seven-week CE, a higher percentage of patients with chronic lower back pain achieved the FRP. Furthermore, Marshall and Murphy demonstrated that a twelve-week exercise training regimen resulted in diminished muscle activity during complete torso flexion [20].
Our results differ from those of previous investigations [40-42]. Shamsi et al. found no significant impact of stretching and strengthening exercises on FRP compared to a control group [43].
Among the inconsistencies in this study and our research, we can identify differences in the types of exercises used, age of the participants, methods employed to measure muscle activity, and positions used for measuring muscle activity. Additionally, in this study, participants reported experiencing pain, whereas in our study, there were no reports of pain.
Additionally, our findings conflict with those of Horn and Bishop [10], who indicated that the acute onset of LBP caused by delayed onset muscle soreness (DOMS) did not influence the fatiguing recovery rate. They suggested that changes in FRR may not be significantly affected by acute pain in back muscles triggered by the delayed onset muscle soreness (DOMS) protocol [42]. CE is a type of exercise therapy designed to improve the coordination between the superficial and deep muscles of the neck, as well as to enhance neuromuscular control in individuals with FHA and RSA postures [16]. The text indicates that altered muscle activity and the creep phenomenon can impact FRP impairment [44, 45]. It highlights that CE contributes to improved posture and helps reestablish a normal balance of muscle activity among agonist and antagonist muscle groups. Additionally, CE increases the elongation capacity of muscle groups that restrict joint movement [18]. Our research produced two significant findings: First, it showed that functional recovery performance can be improved with a specific exercise program; second, it confirmed that the main factor affecting FRP is the insufficient stability of the cervical core. Targeted exercise regimens may enhance FRP by activating the deep cervical muscles and providing the necessary stability to the cervical spine [19].
The outcome of the existing research on the FSA of the participants indicates that the training period had a positive effect. The findings of the present study regarding the FHP and RSP correction of the participants indicate that the training period had a positive effect. The findings of the current study are consistent with those of Abdollahi et al. [46]. Idan Almasoodi et al. [47], and Letafatkar et al. [16], with no discrepancies noted.
FHP and RSP are associated with shortening of the UT, posterior cervical extensor muscles (including the suboccipital, semispinalis, and splenius muscles), SCM, LS, and pectoralis major muscles, as well as weakness of the deep cervical flexors [48].
The useful change mechanisms of posture in the experimental group might have occurred from a combination of enhancements in motor control and neuromuscular efficiency [25, 49, 50], as well as improvements in the deep cervical flexor muscle and scapular realignment involving depression, downcast rotation, and/or abduction (internal rotation) [38]. In the present study, a useful change in posture has been demonstrated. Posture has been demonstrated to be a consequence of integrated change, such as reduced activation of superficial muscles, strengthening of weak muscles during arm motions [25, 49, 50], decreased compressive forces on the cervical epiphyseal joints, and enhanced length and strength of connective tissue [51].
Other possible mechanisms for reducing FHP and forward shoulders in this study include the following: Reducing the activity of the UT, SCM, scalene, and CES muscles; strengthening the cervical deep flexor muscles; and engaging the synergistic muscles in this area [25, 52]. The exercise protocol used in this study was designed to stretch the anterior shoulder muscles and strengthen the posterior shoulder muscles, which may have affected FHP and RSP.
Additionally, strengthening the stabilizing muscles of the scapula and stretching the pectoralis major and minor muscles are effective in reducing FHP and FSP. In this study, to correct the FSAs and forward head situation, we utilized lengthening exercises for the chest muscles and posterior shoulder structures, augmented training for the scapular retractors, serratus anterior, and shoulder rotators, stretching of the LS muscles, strengthening of the deep neck flexors, strengthening of the thoracic spine extensors, and stretching of the anterior structures [25, 53].
Conclusion
TER successfully reverses FRP and improves posture in patients with FHRS. CE programs can help correct potential muscle imbalances that may cause compensatory movements, ultimately resulting in FHA and RSA. The CE therapy outlined in this study aims to enhance regulation among the superficial and deep neck muscles and improve neuromuscular control in individuals with functional headache disorders and recurrent shoulder pain. This approach may help activate underactive deep muscles while reducing strain on the surface muscles. Additionally, the study could validate the primary mechanism behind Functional Rehabilitation Programs and highlight that instability is a recognized contributing factor to this issue.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Council of Hormozgan University of Medical Sciences, Bandar Abbas, Iran (IR.HUMS.REC.1401.417). Trial registration: Iranian Registry of Clinical Trials (IRCT20200622047888N1).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
The authors would like to express their deepest appreciation for the valuable assistance and contributions of all the patients.
References
Today’s teenagers are highly aware of the media and frequently use advanced technologies, such as smartphones [1]. The 2021 report on digital news users in Spain, released by the University of Navarra and University of Oxford, reveals that the cell phone is the predominant device utilized by Internet consumers for information retrieval. Specifically, 90% of users regularly engage with their cell phones for various purposes, and 78% utilize them for news consumption. This represents a five-percentage-point increase compared to 2020 and an 11-point rise from 2019, when the figure stood at 67%. Additionally, insights from the most recent annual report of the national observatory of telecommunications and the information society further support these findings [2]. This constant engagement with devices makes adolescents vulnerable to negative impacts, particularly regarding posture. Extended use of smartphones, laptops, and other screens can lead to common postural issues, such as forward head angle (FHA) and rounded shoulder angle (RSA) [3]. The occurrence of forward head posture (FHP) and rounded shoulder posture (RSP) among a cohort of healthy individuals aged 20-50 years was documented, revealing that 66% exhibited FHP, 73% displayed right RSP, and 66% presented left RSP [4]. In adolescents, the prevalence of common postural abnormalities indicated that the most frequently observed issue was uneven shoulder height (36%), followed by FHP (25%) [4]. Additionally, individuals with FHP demonstrate increased extension of the atlantooccipital joint and upper cervical spine, which is linked to flexion in the lower cervical and upper thoracic spine [5]. RSP is defined by a protruded acromion process of the shoulder joint about the gravitational line, resulting in a stooped posture characterized by elevation, protraction, and downward rotation of the scapula. Furthermore, this condition leads to an increased angle between the lower cervical vertebrae and the upper spine [6].
During computer work, the cervical erector spinae (CES) muscle plays a crucial role in effective activation and support for the task at hand. According to Yoo et al. [6], the fatigue experienced by the CES muscle as a result of tasks involving visual display terminals can be measured using the flexion relaxation phenomenon (FRP). This phenomenon is characterized by a lack of electrical activity in the erector spinae (ES) muscles when the trunk is fully flexed [7, 8]. The FRP occurs because the load shifts from the active muscles (ES) to the passive structures of the spine, such as ligaments, capsules, and intervertebral discs [9, 10]. The FRP observed in the cervical spine mirrored that of the ES muscle. During neck flexion, the cervical extensors gradually increase their activation to manage the increasing load from the head [11]. Once the head is completely flexed, the responsibility of supporting this load shifts from the active muscles to the passive structures, leading to a decrease or cessation of myoelectric activity in the muscles [12]. This interplay between the active and passive components is essential for maintaining the mechanical stability of the spine and its neural system [13].
However, research indicates that these postures, resulting from prolonged neck flexion, place static strain on the musculoskeletal system and increase compressive stress on the cervical spine. Over time, this can lead to detrimental changes in spinal soft tissues and negatively affect neck muscle function [4-6]. The interplay of creep and extended static loading can lead to increased looseness in the lower back, thereby compromising stability of the spinal column [7, 8]. Attaining spinal stability depends on well-coordinated collaboration between the active and passive elements of the neuromusculoskeletal system. In the neck region of the spine, passive stability is provided by the viscoelastic characteristics of the spinal structures [9], whereas active stability arises from both intentional and reflexive muscle activation [9]. Multiple studies examining the lumbar spine have suggested that prolonged trunk flexion leads to a reduction in passive support [10] and that active stiffness is crucial for maintaining spinal stability when passive support is lacking [9].
Researchers investigating the interplay between passive and active stabilizers often utilize FRP [11]. The FRR is the proportion of the highest activation during the re-expansion stage to the mean activation observed during the maximum bending position stage (the quiet period) [12].
This phenomenon explains how muscles and viscoelastic structures, such as ligaments, disks, capsules, and fascia, collaborate to distribute loads effectively [13]. As neck flexion occurs, cervical extensors progressively enhance their activation to counteract the gravitational pull on the head’s position. When the completely bent position, the stressed viscoelastic structures generate an encumbrance enough to counteract gravity, resulting in decreased activation of the extensor muscles [14]. Some studies have noted the lack or postponement of FRP during full neck flexion, especially among participants experiencing neck pain [15].
Abnormal flexion-relaxation patterns can be improved through exercise interventions [16, 17], which may also help rectify muscle imbalances that lead to movement compensations associated with Letafatkar et al. [16] For example, deep cervical flexor strengthening exercises are advised to mitigate FH and RS and promote an upright posture [18]. Many studies have examined how exercise affects flexion-relaxation patterns in people experiencing low back pain [16, 19], and FH and RS [20]; however, there is a deficiency of substantial information and consensus regarding the influence of therapeutic exercise routines on these patterns. Some therapies have demonstrated no effect on the flexion-relaxation response (FRP) [20-22], whereas other studies have indicated an improvement in the flexion-relaxation pattern following therapeutic exercise [23, 24].
A therapeutic exercise program was created to focus on alterations in posture, center of gravity, and base of support in the three participants. The regimen was divided into three stages: The initial stage focused on slow, controlled movements to alleviate pain, enhance muscle collaboration, and improve proprioception; the secondary stage aimed at building muscular endurance; and the final stage emphasized muscle strengthening. The participants were instructed to perform each exercise for approximately 30-60 s. Furthermore, the program provided instructions on how to realign the spine, scapula, glenohumeral joint, cervical, and stomach during each meeting, highlighting the importance of maintaining these alignments against a wall or bed whenever possible before starting the therapeutic exercises.
The procedure assists in preventing deviation of neck and waist lordosis, as well as roundback, while performing exercises [25]. However, to our knowledge, no study with random assignment to groups (randomized controlled trials [RCT]) has examined the effectiveness of corrective exercise (CE) on the FRP in participants with flexion-related symptomatic postural impairments. Additionally, evidence regarding the impact of CE on FRP is limited and lacks consensus; to date, no studies have utilized CE to enhance flexion-relaxation. Moreover, these exercises require no special equipment or facilities and can be easily performed at home.
The target was to ascertain the benefits of training on FRP and posture in individuals with FHP and RSP. We hypothesized that CE would improve flexion-relaxation and postural misalignments in individuals with FHA and RSA postures after 8 weeks. The control group did not undergo training and engaged in their regular daily activities.
Materials and Methods
A randomized controlled trial (RCT) was conducted, and ethical clearance was obtained from the Ethics Committee of Hormozgan University of Medical Sciences. Initially, 80 participants were recruited from a university physical therapy clinic that serves clients from the surrounding community. The yardstick for involvement is among 15- to 20-year-olds, having a body mass index (BMI) of 20-25 kg/m², a forward shoulder angle (FSA) exceeding 52°, and an FHA greater than 46°, with these angles measured through photogrammetry (Figure 1). Subjects were omitted if they had a chronicle of cervical spine or back surgery, exhibited neurological symptoms, suffered from atrophic arthritis impacting the neck or back, were currently taking muscle relaxants, engaged in regular physical activity each week, or were professional athletes [26], non-completion of the training program according to the research, lack of willingness of participants to continue participating in the research, non-participation of the participants in two consecutive training sessions, injury during the execution of the exercises [27, 28]. Next, applying the yardstick for involvement and elimination, an expert in physical therapy selected 60 participants. The sample size was established based on preliminary analysis utilizing G*Power software, with the FHA score serving as the primary outcome variable. (Figure 1). Assessments were performed at the beginning of the research and again after 8 weeks at the university’s physical therapy clinic. Following the initial assessment, participants were allocated to one of two groups: Group 1 (CE) and Group 2 (Control). Group 1 underwent a supervised intervention for 8 weeks, while the control group carried out their daily activities. Randomization was implemented using a computerized random number generator. The allocation sequence was kept hidden from the researcher responsible for enrolling and evaluating participants using sequentially numbered, opaque, sealed envelopes. Participants were partially blinded, as they did not know the expected diversity among the groups, but were aware of the treatment they were receiving.

The BioPrint system for postural analysis was utilized to assess posture (Biotonix Inc., Montreal, CA). Markers were affixed to specific anatomical points, including the right tragus of the ear, acromion process, and C7 spinous process. The participants were then guided to position themselves 40 cm from a backdrop, perform three forward bends, reach overhead three times, and ultimately stand upright while looking directly ahead in their usual sleeping position. A digital camera (Canon Power Shot 95, USA) was mounted on a 1-meter-high tripod, positioned 3.5 m from the wall. Photographs were captured from the right side of the participants in sagittal sitting posture. Measurements of FHA and FSA were obtained utilize photo processing software (Adobe Photoshop) as follows: FHA was determined from the vertical anterior line connecting the tragus and the C7 label, while FSA was assessed from the upright posterior line associated with the C7 marker and the acromial label. Normative data indicate that an FSA greater than 52° suggests RSA, and an FHA greater than 46° indicates FHA [18].
In a cervical flexion-relaxation experiment, cervical extensor muscle activity was recorded using electromyography (EMG) during full cervical flexion (Figure 2). The participants started in a vertical, normal cervical spine position, flexed to their maximum extent, and then returned to the normal position. While executing this task, the participants sat vertically on a stool, with their hips and knees at 90° and feet resting on the floor shoulder-width apart. Their shoulders were aligned with their trunk at approximately 90° internal rotation, and their forearms were pronated, with hands relaxed on their hips. The cervical flexion-relaxation procedure consisted of five stages, each lasting three seconds: Phase 1 involved maintaining a normal neck position; phase two required uttermost neck flexion; phase 3 was a hold at maximal cervical flexion; stage four was cervical extension back to the neutral position; and stage five was a hold at the common position [29]. To ensure consistency in speed and duration across all phases, the assistant counted in synchrony with a metronome set to one beat per second [30]. The participants were instructed to maintain a steady, vertical trunk posture to avoid bending or tilting, and to focus on a fixed point directly ahead to maintain the initial head position. Before the flexion-relaxation task, participants practiced with the metronome and the rhythm of head motions until they could consistently perform the task. These tasks were performed three times in succession without breaks between sets.

EMG
In the electrode placement stage, disposable surface electrodes (models SKINTACT, made in Austria) were used. The center-to-center distance between the electrodes was approximately two and a half centimeters. Initially, the skin was shaved and sanded to decrease skin resistance and improve the quality of the received surface electromyographic (SEMG) signals, and it was cleaned with 70% alcohol. Additionally, the electrodes were placed along the orientation of the muscle fibers by the SENIAM guidelines.
After shaving and polishing the skin at the electrode sites, surface EMG signals were captured from four muscles using a Bagnoli-16 system (FREE EMG 300, BTS Bioengineering, Italy) with a sampling rate of 1000 Hz and a bandwidth of 20–450 Hz. Four pairs of active single-differential dry surface electrodes were evenly positioned on the sternocleidomastoid (SCM), upper trapezius (UT), ES, and levator scapulae (LS) muscles, in line with the established placement protocols (Figure 2). For the UT muscle, the electrode is positioned bilaterally between the spinous process of the C7 spine and the acromion [31]. The SCM electrodes are placed near a point that is thirty percent of the distance from the sternal notch to the mastoid process, straight over the muscle belly of the sternal head [32]. The electrodes for the ES are located 2 cm lateral to the spinous processes of C4 and C5 [32]. In contrast, the electrodes for the LS are positioned betwixt the anterior border of the UT and the posterior border of the SCM [31].
The functional muscle proportion was determined by measuring the greatest muscular activity (one-secondary root mean square [RMS]) of every muscle while in a flexed head position and then dividing this value by the one-second RMS of greatest voluntary contraction for the corresponding muscle.
The entire process of analyzing EMG signals was carried out using MATLAB software. First, EMG data were recorded and stored using a wireless device of brand 16 with a sampling frequency of 1000 Hz. Then, the statistics noise was filtered with a bandwidth of 10 to 450 Hz. The recorded data were analyzed using the RMS method to determine the level of activity. To normalize the data, the activity of every muscle was expressed as a percentage of the maximum RMS during normal activity.
Interventions protocol
The intervention group underwent an 8-week therapeutic exercise routine (Table 1). The exercises were performed twice a week for approximately 20–30 minutes [18]. The intensity of the CE was set to a rating of perceived exertion (RPE) of 11–13 (RPE, Borg’s 6–20 scale), which corresponds to a light-to-somewhat hard training intensity [32]. Most exercises were planned according to the TER principles, with each exercise targeting posture, the focal point of stability and the support foundation. The training regimen included exercises designed to strengthen and stretch the muscles. Exercise advancement was developed in accordance with the findings of an earlier study [33].

The intervention consisted of three distinct phases. Every initial phase focused on performing slow and controlled training that caused little discomfort, aimed at enhancing muscle coordination and proprioception. The secondary period prioritizes building muscular endurance. The final stage concentrated on muscle reinforcement [33]. Each training session was performed for a duration of 30-60 s [16]. During the second stage, the participants were required to complete three sets of 15 reiterations, with the initial 12 reiterations performed at maximum force, allowing for a 1-minute rest between sets. In the third phase, the participants were encouraged to perform as many reiterations as possible, targeting three sets of 15 reiterations. The control group received advice on how to correct posture. This advice included the following: 1) Reduce neck extension and forward movement of the neck while performing daily tasks. 2) While sitting at the computer, have a supportive chair that will decrease thoracic flexion and help conserve good thoracic posture [16]: 3) Support your forearms either on the desk or an extended tray for a keyboard. The desk or tray should be at the appropriate height, so you do not need to “slouch” for your arms to be supported; 4) Alignment correction when wearing glasses should follow the same sequence that has been demonstrated in the sitting back-to-wall exercises: Start with correction of lumbar, thoracic, and scapular alignment, and then neck and head position; 5) During daily activities, amend your alignment and decrease the stress imposed by adjacent joints before initiating cervical movements [16], and particularly correct the position of your thoracic vertebrae and shoulder girdle, and support your upper limbs; 6) Apply your abdominals to maintain normal lumbar spine alignment and prevent thoracic flexion or “slumping”, particularly when sitting [16].
Statistical analysis
Data were analyzed using the SPSS software, version 21.0 (IBM Corporation, Chicago, IL). To assess the normality of the data, a Kolmogorov-Smirnov test was employed. A 2×2 mixed repeated measures design was implemented to evaluate and compare changes over time, and determine whether these changes varied between the control and TER groups. The significance level was set at <0.05. Effect sizes and 95% confidence intervals (CIs) were calculated to assess clinical significance.
Results
The two groups were similar at the beginning of the study because no significant differences were observed (P>0.05) in their demographic and clinical characteristics (Table 2).

Treatment effects
There were main effects of time (P<0.001) and group (P<0.001), as well as a group×time interaction (P<0.001) for FHA, RSA, start and end of eccentric contraction, start and end of concentric contraction, SCM, LS, RMS, UT, and ES. These interactions indicate that the changes over the 8 weeks differed between the control and CE groups.
Only in the CE, but not in the control group, the FHA (P<0.001, ES=0.58, 95% CI, 1.10%, 0.06%) and RSA (P<0.001, ES=0.68, 95% CI, -4.94%, -2.83%) were reduced.
In the FE task, the participants in the CE group observed significantly less RSA (P<0.001, ES=0.68, 95% CI, -4.49%, -2.83%).
In the FE task, participants in the CE group demonstrated a significantly earlier onset of eccentric contraction after the intervention than those in the control group (P<0.001, ES=0.58, 95% CI, -1.10%, 0.06%). Additionally, the participants in the experimental group also demonstrated a significantly earlier cessation of eccentric contraction post-intervention than those in the control group (P=0.000, ES=0.91, 95% CI, -1.44%, -0.37%). Furthermore, the CE group exhibited a significantly delayed onset of concentric contraction after the intervention compared to the control group (P=0.000, ES=0.51, 95% CI, -0.00%, -1.02%). Lastly, the CE group showed a significantly earlier end of concentric contraction post-intervention than to the control group (P=0.001, ES=0.35, 95% CI, -0.86%, -0.15%).
In the FE task, participants in the CE group demonstrated a significantly earlier onset for UT at the post-intervention stage compared to the control group (P<0.001), with an effect size of 1.47 (95% CI, -2.04%, -0.90%). Similarly, for SCM, the TER group also demonstrated a significantly earlier onset post-intervention relative to the control group (P<0.001), with an effect size of 1.23 (95% CI, -1.78%, -0.67%). Additionally, the CE group exhibited a significantly earlier onset for ES (P<0.003), with an effect size of 1.23 (95% CI, -1.23%, -0.19%). For LS, the CE group again showed a significantly earlier onset later-intervention compared to the control group (P=0.000), with an effect size of 1.14 (95% CI, -1.68%, -0.59%). While there was a significant main effect of time (P<0.000), there was no significant group effect (P<0.189) or interaction among group and time for onset, UT (P<0.152).
In the FE task, participants in the CE group demonstrated a significantly earlier RMS for UT at post-intervention compared to the control group (P<0.001), with an effect size (ES) of 0.69 (95% CI, -1.73%, 0.34%). Similarly, the CE group showed a significantly earlier RMS for SCM (P<0.001), with an ES of 1.57 (95% CI, -2.15%, -0.99%). There was a significant main effect of time (P<0.001) and group (P<0.001), along with a notable interaction between group and time on RMS and UT (P<0.001). Additionally, the CE group exhibited a significantly earlier RMS for ES (P<0.001), with an ES of 0.44 (95% CI, -1.47%, 0.57%), and LS (P<0.001), with an ES of 0.49 (95% CI, -1.51%, 0.53%) (Tables 3, 4 and 5).



Discussion
This study aimed to investigate the effects ofCE on FRP and posture in individuals with flexion-related postural dysfunction. The results showed that participants in the CE group showed notable enhancements in both FRP and posture after an eight-week exercise program. The intervention group showed an improvement in the craniovertebral and shoulder angles after training, while the control group did not exhibit any notable changes in these angles. Studies have repeatedly indicated that individuals with forward head and FSA (FHRSA) frequently display irregular flexion-relaxation patterns. The findings of this study confirm that the irregular FRP observed in individuals with FHRS can be significantly improved by applying TER focused on the cervical spine, leading to better FRP outcomes.
Many studies have investigated how stretching programs can enhance range of motion [34, 35]. Certain evaluations suggest that engaging in strength training while the muscle is in an extended position may lead to structural changes [36]. Strength training leads to an enlargement of the muscle’s cross-sectional area by increasing the number of parallel sarcomeres. Additionally, this type of exercise modifies the number of serial sarcomeres, which in turn influences muscle length. The specific length at which muscles are activated during strength training is crucial. Our results are consistent with numerous previous studies [20, 37]. Mak et al. conducted a study on the functional recovery rate (FRR) associated with bending from a seated position in individuals suffering from low back pain (LBP) after undergoing a rehabilitation program. Their findings indicated an improvement in FRR, which was determined by calculating the ratio of RMS values in an upright sitting position to those in a flexed sitting position. A significant rise in the FRR was observed in LBP patients when comparing their status before and after rehabilitation; however, our method indicated a reduction (P<0.05) [18]. One reason for the inconsistency between the results of this study and our research is that our study focused on adolescents, whereas this study focused on adults. Additionally, our research sample included only of boys, while this study included both men and women. Furthermore, the research conducted by Mak et al. measured lumbar muscle flexion relaxation, whereas our study measured neck muscle flexion relaxation. Moreover, the positions used to measure muscle activity differed between the two studies. In our study, the movement involved the neck, while in the study by Mak at al., the assessment was conducted in a sitting position with bending movements and returning from a bent position to an upright sitting position [18].
Furthermore, Neblett et al. investigated variations in FRRs in a cohort of 54 patients with LBP both before and after a back rehabilitation program [38]. The treatment strategy included counseling for stress management and SEMG biofeedback to facilitate relaxation of trunk muscles during flexion. Following treatment, the proportion of patients with chronic low back pain (CLBP) who exhibited normal functional recovery patterns increased significantly, from 30% to 95%. Notably, this study indicated that exercise could effectively normalize abnormal FRP [39]. However, our method indicated a reduction (P<0.05).
One reason for the discrepancies between the results of this study and those of Neblett et al. [38] is that the exercises performed in their study differed from those in our research. Additionally, in the study above, the participants experienced back pain, whereas in our study, the individuals had FHP and forward shoulder posture (FSP). Furthermore, the methods used to measure muscle activity differed between studies.
In addition, Park and Choi examined how stabilization training influences FRP in the erector spinae muscles. Their outcomes suggest that lumbar stabilization training can alleviate FRP asymmetry in these muscles, potentially decreasing the incidence of low back pain in the general population [37]. Our research supports their findings, indicating that following a seven-week CE, a higher percentage of patients with chronic lower back pain achieved the FRP. Furthermore, Marshall and Murphy demonstrated that a twelve-week exercise training regimen resulted in diminished muscle activity during complete torso flexion [20].
Our results differ from those of previous investigations [40-42]. Shamsi et al. found no significant impact of stretching and strengthening exercises on FRP compared to a control group [43].
Among the inconsistencies in this study and our research, we can identify differences in the types of exercises used, age of the participants, methods employed to measure muscle activity, and positions used for measuring muscle activity. Additionally, in this study, participants reported experiencing pain, whereas in our study, there were no reports of pain.
Additionally, our findings conflict with those of Horn and Bishop [10], who indicated that the acute onset of LBP caused by delayed onset muscle soreness (DOMS) did not influence the fatiguing recovery rate. They suggested that changes in FRR may not be significantly affected by acute pain in back muscles triggered by the delayed onset muscle soreness (DOMS) protocol [42]. CE is a type of exercise therapy designed to improve the coordination between the superficial and deep muscles of the neck, as well as to enhance neuromuscular control in individuals with FHA and RSA postures [16]. The text indicates that altered muscle activity and the creep phenomenon can impact FRP impairment [44, 45]. It highlights that CE contributes to improved posture and helps reestablish a normal balance of muscle activity among agonist and antagonist muscle groups. Additionally, CE increases the elongation capacity of muscle groups that restrict joint movement [18]. Our research produced two significant findings: First, it showed that functional recovery performance can be improved with a specific exercise program; second, it confirmed that the main factor affecting FRP is the insufficient stability of the cervical core. Targeted exercise regimens may enhance FRP by activating the deep cervical muscles and providing the necessary stability to the cervical spine [19].
The outcome of the existing research on the FSA of the participants indicates that the training period had a positive effect. The findings of the present study regarding the FHP and RSP correction of the participants indicate that the training period had a positive effect. The findings of the current study are consistent with those of Abdollahi et al. [46]. Idan Almasoodi et al. [47], and Letafatkar et al. [16], with no discrepancies noted.
FHP and RSP are associated with shortening of the UT, posterior cervical extensor muscles (including the suboccipital, semispinalis, and splenius muscles), SCM, LS, and pectoralis major muscles, as well as weakness of the deep cervical flexors [48].
The useful change mechanisms of posture in the experimental group might have occurred from a combination of enhancements in motor control and neuromuscular efficiency [25, 49, 50], as well as improvements in the deep cervical flexor muscle and scapular realignment involving depression, downcast rotation, and/or abduction (internal rotation) [38]. In the present study, a useful change in posture has been demonstrated. Posture has been demonstrated to be a consequence of integrated change, such as reduced activation of superficial muscles, strengthening of weak muscles during arm motions [25, 49, 50], decreased compressive forces on the cervical epiphyseal joints, and enhanced length and strength of connective tissue [51].
Other possible mechanisms for reducing FHP and forward shoulders in this study include the following: Reducing the activity of the UT, SCM, scalene, and CES muscles; strengthening the cervical deep flexor muscles; and engaging the synergistic muscles in this area [25, 52]. The exercise protocol used in this study was designed to stretch the anterior shoulder muscles and strengthen the posterior shoulder muscles, which may have affected FHP and RSP.
Additionally, strengthening the stabilizing muscles of the scapula and stretching the pectoralis major and minor muscles are effective in reducing FHP and FSP. In this study, to correct the FSAs and forward head situation, we utilized lengthening exercises for the chest muscles and posterior shoulder structures, augmented training for the scapular retractors, serratus anterior, and shoulder rotators, stretching of the LS muscles, strengthening of the deep neck flexors, strengthening of the thoracic spine extensors, and stretching of the anterior structures [25, 53].
Conclusion
TER successfully reverses FRP and improves posture in patients with FHRS. CE programs can help correct potential muscle imbalances that may cause compensatory movements, ultimately resulting in FHA and RSA. The CE therapy outlined in this study aims to enhance regulation among the superficial and deep neck muscles and improve neuromuscular control in individuals with functional headache disorders and recurrent shoulder pain. This approach may help activate underactive deep muscles while reducing strain on the surface muscles. Additionally, the study could validate the primary mechanism behind Functional Rehabilitation Programs and highlight that instability is a recognized contributing factor to this issue.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Council of Hormozgan University of Medical Sciences, Bandar Abbas, Iran (IR.HUMS.REC.1401.417). Trial registration: Iranian Registry of Clinical Trials (IRCT20200622047888N1).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
The authors would like to express their deepest appreciation for the valuable assistance and contributions of all the patients.
References
- Alizadeh S, Daneshjoo A, Zahiri A, Anvar SH, Goudini R, Hicks JP, et al. Resistance training induces improvements in range of motion: A systematic review and meta-analysis. Sports Medicine. 2023; 53(3):707-22. [PMID]
- Järvi T. Analyzing digital transition in small and medium sized companies in Spain [BA thesis]. Helsinki: Haaga-Helia University of Applied Sciences; 2022. [Link]
- Borna L, Ahmadi A, Sarafzadeh J, Maarufi N. [Comparison of cervical flexion relaxation phenomenon between forward head posture and healthy subjects (Persian)]. Journal of Modern Rehabilitation. 2016; 9(S3):65-71. [Link]
- Mosaad DM, Abdel-aziem AA, Mohamed GI, Abd-Elaty EA, Mohammed KS. Effect of forward head and rounded shoulder posture on hand grip strength in asymptomatic young adults: a cross-sectional study. Bulletin of Faculty of Physical Therapy. 2020; 25(1):1-8. [DOI:10.1186/s43161-020-00001-z]
- Dalbudak E, Evren C, Aldemir S, Coskun KS, Ugurlu H, Yildirim FG. Relationship of internet addiction severity with depression, anxiety, and alexithymia, temperament and character in university students. Cyberpsychology, Behavior, and Social Networking. 2013; 16(4):272-8. [DOI:10.1089/cyber.2012.0390] [PMID]
- Yoo WG, Yi CH, Kim MH. Effects of a proximity-sensing feedback chair on head, shoulder, and trunk postures when working at a visual display terminal. Journal of Occupational Rehabilitation. 2006; 16(4):631-7. [DOI:10.1007/s10926-006-9059-7]
- Forbes PA, de Bruijn E, Schouten AC, van der Helm FC, Happee R. Dependency of human neck reflex responses on the bandwidth of pseudorandom anterior-posterior torso perturbations. Experimental Brain Research. 2013; 226(1):1-14. [DOI:10.1007/s00221-012-3388-x] [PMID]
- Grimmer K. An investigation of poor cervical resting posture. The Australian Journal of Physiotherapy. 1997; 43(1):7-16. [DOI:10.1016/S0004-9514(14)60398-6] [PMID]
- Grimmer-Somers K, Milanese S, Louw Q. Measurement of cervical posture in the sagittal plane. Journal of Manipulative and Physiological Therapeutics. 2008; 31(7):509-17. [DOI:10.1016/j.jmpt.2008.08.005] [PMID]
- Horn ME, Bishop MD. Flexion relaxation ratio not responsive to acutely induced low back pain from a Delayed Onset Muscle Soreness Protocol. ISRN Pain. 2013; 2013:617698. [DOI:10.1155/2013/617698] [PMID]
- Janwantanakul P, Sitthipornvorakul E, Paksaichol A. Risk factors for the onset of nonspecific low back pain in office workers: A systematic review of prospective cohort studies. Journal of Manipulative and Physiological Therapeutics. 2012; 35(7):568-77. [DOI:10.1016/j.jmpt.2012.07.008] [PMID]
- Nimbarte AD, Zreiqat MM, Chowdhury SK. Cervical flexion-relaxation response to neck muscle fatigue in males and females. Journal of Electromyography and Kinesiology. 2014; 24(6):965-71. [DOI:10.1016/j.jelekin.2014.09.002] [PMID]
- Choi KH, Cho MU, Park CW, Kim SY, Kim MJ, Hong B, et al. A comparison study of posture and fatigue of neck according to monitor types (moving and fixed monitor) by using flexion relaxation phenomenon (FRP) and craniovertebral angle (CVA). International Journal of Environmental Research and Public Health. 2020; 17(17):6345. [DOI:10.3390/ijerph17176345] [PMID]
- Kim D, Lee Y, Lee J, Nam JK, Chung Y. Development of Korean smartphone addiction proneness scale for youth. PLoS One. 2014; 9(5):e97920. [DOI:10.1371/journal.pone.0097920] [PMID]
- Lau KT, Cheung KY, Chan MH, Lo KY, Chiu TT. Relationships between sagittal postures of thoracic and cervical spine, presence of neck pain, neck pain severity and disability. Manual Therapy. 2010; 15(5):457-62. [DOI:10.1016/j.math.2010.03.009] [PMID]
- Letafatkar A, Rabiei P, Alamooti G, Bertozzi L, Farivar N, Afshari M. Effect of therapeutic exercise routine on pain, disability, posture, and health status in dentists with chronic neck pain: A randomized controlled trial.International Archives of Occupational and Environmental Health. 2020; 93(3):281-90. [DOI:10.1007/s00420-019-01480-x] [PMID]
- Little JS, Khalsa PS. Human lumbar spine creep during cyclic and static flexion: Creep rate, biomechanics, and facet joint capsule strain. Annals of Biomedical Engineering. 2005; 33(3):391-401. [DOI:10.1007/s10439-005-1742-x] [PMID]
- Mak JN, Hu Y, Cheng AC, Kwok HY, Chen YH, Luk KD. Flexion-relaxation ratio in sitting: Application in low back pain rehabilitation. Spine. 2010; 35(16):1532-8. [DOI:10.1097/BRS.0b013e3181ba021e] [PMID]
- Mannion AF, Junge A, Taimela S, Müntener M, Lorenzo K, Dvorak J. Active therapy for chronic low back pain: Part 3. Factors influencing self-rated disability and its change following therapy. Spine. 2001; 26(8):920-9. [DOI:10.1097/00007632-200104150-00015] [PMID]
- Marshall P, Murphy B. Changes in the flexion relaxation response following an exercise intervention. Spine. 2006; 31(23):E877-E83. [DOI:10.1097/01.brs.0000244557.56735.05] [PMID]
- Meyer JJ, Berk RJ, Anderson AV. Recruitment patterns in the cervical paraspinal muscles during cervical forward flexion: Evidence of cervical flexion-relaxation. Electromyography and Clinical Neurophysiology. 1993; 33(4):217-23. [PMID]
- Mousavi-Khatir R, Talebian S, Maroufi N, Olyaei GR. Effect of static neck flexion in cervical flexion-relaxation phenomenon in healthy males and females. Journal of Bodywork and Movement Therapies. 2016; 20(2):235-42. [DOI:10.1016/j.jbmt.2015.07.039] [PMID]
- Neblett R, Mayer TG, Gatchel RJ, Keeley J, Proctor T, Anagnostis C. Quantifying the lumbar flexion-relaxation phenomenon: Theory, normative data, and clinical applications. Spine (Phila Pa 1976). 2003; 28(13):1435-46. [DOI:10.1097/01.BRS.0000067085.46840.5A] [PMID]
- Netto KJ, Burnett AF. Reliability of normalisation methods for EMG analysis of neck muscles. Work. 2006; 26(2):123-30. [DOI:10.3233/WOR-2006-00500]
- Sánchez-Zuriaga D, Adams MA, Dolan P. Is activation of the back muscles impaired by creep or muscle fatigue? Spine. 2010; 35(5):517-25. [DOI:10.1097/BRS.0b013e3181b967ea] [PMID]
- Ritvanen T, Zaproudina N, Nissen M, Leinonen V, Hänninen O. Dynamic surface electromyographic responses in chronic low back pain treated by traditional bone setting and conventional physical therapy. Journal of Manipulative and Physiological Therapeutics. 2007; 30(1):31-7. [DOI:10.1016/j.jmpt.2006.11.010] [PMID]
- Dingenen B, Malfait B, Vanrenterghem J, Robinson MA, Verschueren SM, Staes FF. Can two-dimensional measured peak sagittal plane excursions during drop vertical jumps help identify three-dimensional measured joint moments? The Knee. 2015; 22(2):73-9. [DOI:10.1016/j.knee.2014.12.006] [PMID]
- Dingenen B, Malfait B, Vanrenterghem J, Verschueren SM, Staes FF. The reliability and validity of the measurement of lateral trunk motion in two-dimensional video analysis during unipodal functional screening tests in elite female athletes. Physical Therapy in Sport. 2014; 15(2):117-23. [DOI:10.1016/j.ptsp.2013.05.001] [PMID]
- Ruggiero SA, Frost LR, Vallis LA, Brown SH. Effect of short-term application of kinesio tape on the flexion-relaxation phenomenon, trunk postural control and trunk repositioning in healthy females. Journal of Sports Sciences. 2016; 34(9):862-70. [DOI:10.1080/02640414.2015.1076164] [PMID]
- Ruivo RM, Pezarat-Correia P, Carita AI. Effects of a resistance and stretching training program on forward head and protracted shoulder posture in adolescents. Journal of Manipulative and Physiological Therapeutics. 2017; 40(1):1-10.[DOI:10.1016/j.jmpt.2016.10.005] [PMID]
- Girasol CE, Dibai-Filho AV, de Oliveira AK, de Jesus Guirro RR. Correlation between skin temperature over myofascial trigger points in the upper trapezius muscle and range of motion, electromyographic activity, and pain in chronic neck pain patients. Journal of Manipulative and Physiological Therapeutics. 2018; 41(4):350-7. [DOI:10.1016/j.jmpt.2017.10.009]
- Lascurain-Aguirrebeña I, Newham DJ, Irazusta J, Seco J, Critchley DJ. Reliability of a method to measure neck surface electromyography, kinematics, and pain occurrence in participants with neck pain. Journal of Manipulative and Physiological Therapeutics. 2018; 41(5):413-24. [DOI:10.1016/j.jmpt.2017.10.013]
- Shahvarpour A, Henry SM, Preuss R, Mecheri H, Larivière C. The effect of an 8-week stabilization exercise program on the lumbopelvic rhythm and flexion-relaxation phenomenon. Clinical Biomechanics. 2017; 48:1-8. [DOI:10.1016/j.clinbiomech.2017.06.010] [PMID]
- Afonso J, Ramirez-Campillo R, Moscão J, Rocha T, Zacca R, Martins A, et al. Strength training is as effective as stretching for improving range of motion: A systematic review and meta-analysis. Healthcare. 2021; 9(4):427. [DOI:10.3390/healthcare9040427]
- Sheikhhoseini R, Shahrbanian S, Sayyadi P, O'Sullivan K. Effectiveness of therapeutic exercise on forward head posture: A systematic review and meta-analysis. Journal of Manipulative and Physiological Therapeutics. 2018; 41(6):530-9. [DOI:10.1016/j.jmpt.2018.02.002] [PMID]
- Shin G, Mirka GA. An in vivo assessment of the low back response to prolonged flexion: Interplay between active and passive tissues. Clinical Biomechanics. 2007; 22(9):965-71. [DOI:10.1016/j.clinbiomech.2007.06.003] [PMID]
- Park SS, Choi BR. Effects of lumbar stabilization exercises on the flexion-relaxation phenomenon of the erector spinae. Journal of Physical Therapy Science. 2016; 28(6):1709-11. [DOI:10.1589/jpts.28.1709] [PMID]
- Neblett R, Mayer TG, Brede E, Gatchel RJ. Correcting abnormal flexion-relaxation in chronic lumbar pain: Responsiveness to a new biofeedback training protocol. The Clinical Journal of Pain. 2010; 26(5):403-9. [DOI:10.1097/AJP.0b013e3181d2bd8c] [PMID]
- Shiravi S, Letafatkar A, Bertozzi L, Pillastrini P, Khaleghi Tazji M. Efficacy of abdominal control feedback and scapula stabilization exercises in participants with forward head, round shoulder postures and neck movement impairment. Sports Health. 2019; 11(3):272-9. [DOI:10.1177/1941738119835223] [PMID]
- Solomonow M, Baratta RV, Banks A, Freudenberger C, Zhou BH. Flexion-relaxation response to static lumbar flexion in males and females. Clinical Biomechanics. 2003; 18(4):273-9. [DOI:10.1016/S0268-0033(03)00024-X] [PMID]
- Simoneau M, Denninger M, Hain TC. Role of loading on head stability and effective neck stiffness and viscosity. Journal of Biomechanics. 2008; 41(10):2097-103. [DOI:10.1016/j.jbiomech.2008.05.002] [PMID]
- Solomonow M, Baratta R, Zhou BH, Burger E, Zieske A, Gedalia A. Muscular dysfunction elicited by creep of lumbar viscoelastic tissue. Journal of Electromyography and Kinesiology. 2003; 13(4):381-96. [DOI:10.1016/S1050-6411(03)00045-2] [PMID]
- Shamsi M, Ahmadi A, Mirzaei M, Jaberzadeh S. Effects of static stretching and strengthening exercises on flexion relaxation ratio in patients with LBP: A randomized clinical trial. Journal of Bodywork and Movement Therapies. 2022; 30:196-202. [DOI:10.1016/j.jbmt.2022.02.023] [PMID]
- Szeto GP, Straker L, Raine S. A field comparison of neck and shoulder postures in symptomatic and asymptomatic office workers. Applied Ergonomics. 2002; 33(1):75-84. [DOI:10.1016/S0003-6870(01)00043-6] [PMID]
- Thigpen CA, Padua DA, Michener LA, Guskiewicz K, Giuliani C, Keener JD, et al. Head and shoulder posture affect scapular mechanics and muscle activity in overhead tasks. Journal of Electromyography and Kinesiology. 2010; 20(4):701-9. [DOI:10.1016/j.jelekin.2009.12.003] [PMID]
- Abdollahi Z, Ghanizadehhesar N, Roshani S, Mohammad Ali Nasab Firouzjah E. [The effect of functional corrective, central stability exercises and combination on the shoulder girdle posture of adolescent girls (Persian)]. Iranian Journal of Rehabilitation Research. 2022; 8(2):42-51. [DOI:10.22034/IJRN.8.2.5]
- Idan Almasoodi MC, Mahdavinejad R, Ghasmi G. The effect of 8 weeks national academy of sports medicine exercises training on posture, shoulder pain, and functional disability in male with upper cross syndrome. Systematic Reviews in Pharmacy. 2020; 11(11):1826-33. [Link]
- Lynch SS, Thigpen CA, Mihalik JP, Prentice WE, Padua D. The effects of an exercise intervention on forward head and rounded shoulder postures in elite swimmers. British Journal of Sports Medicine. 2010; 44(5):376-81. [DOI:10.1136/bjsm.2009.066837]
- Dareh-Deh HR, Hadadnezhad M, Letafatkar A, Peolsson A. Therapeutic routine with respiratory exercises improves posture, muscle activity, and respiratory pattern of patients with neck pain: A randomized controlled trial. Scientific Reports. 2022; 12(1):4149. [DOI:10.1038/s41598-022-08128-w]
- Beneka A, Malliou P, Gioftsidou A. Neck pain and office workers: An exercise program for the workplace. ACSM's Health & Fitness Journal. 2014; 18(3):18-24. [DOI:10.1249/FIT.0000000000000034]
- Silva AG, Punt TD, Sharples P, Vilas-Boas JP, Johnson MI. Head posture and neck pain of chronic nontraumatic origin: A comparison between patients and pain-free persons. Archives of Physical Medicine and Rehabilitation. 2009; 90(4):669-74. [DOI:10.1016/j.apmr.2008.10.018]
- Akodu A, Ipinnimo O, Osuntoki A. Effect of smartphone application on pain, disability and forward head posture on young adults with excessive usage of a smartphone: a pilot study. South African Journal of Public Health. 2023; 7(1):e1175. [DOI:10.7196/SAJPH.2023.v7.i1.1175]
- Sakinepoor A, Hadadnezhad M, Shojaedin SS, Letafatkar A, Khaleghi Tazji M. [Effect of corrective exercises on posture, upper extremity muscle activity, and shoulder kinematics in male handball players with upper cross syndrome (Persian)]. The Scientific Journal of Rehabilitation Medicine. 2024; 12(6):1080-97. [DOI:10.32598/SJRM.12.6.2980]
Type of Study: Research |
Subject:
Sport injury and corrective exercises
Received: 2024/09/19 | Accepted: 2025/01/4 | Published: 2025/10/18
Received: 2024/09/19 | Accepted: 2025/01/4 | Published: 2025/10/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



