Tue, Aug 5, 2025
Volume 15, Issue 3 (Summer 2025)
PTJ 2025, 15(3): 231-242 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Alarab A, Hroub N, Abu Shameh R. Comparative Impact of Aerobic vs Resistance Training on Fatigue, Mobility, and Disability in Cancer Patients. PTJ 2025; 15 (3) :231-242
URL: http://ptj.uswr.ac.ir/article-1-704-en.html
URL: http://ptj.uswr.ac.ir/article-1-704-en.html
1- Department of Physiotherapy, Faculty of Post Graduated Studies and Research, Palestine Ahliya University, Bethlehem, Palestine.
Full-Text [PDF 1649 kb]
(139 Downloads)
| Abstract (HTML) (864 Views)
.PNG)
.PNG)
.PNG)

The sample size was determined based on Cohen’s mathematical technique for sample size computation. The minimum sample size for each group was determined using the Equation 1:
.PNG)
Equation 1 includes the following variables: n denotes the necessary number of participants per group, N represents the total number of groups, Zα is the Z-value that corresponds to the desired level of statistical significance (typically set at 1.96 for a 95% confidence interval [CI]), Zβ is the Z-value that corresponds to the desired power level (typically set at 0.90 for 80% statistical power), and d represents the effect size. The selection of an 80% power level and a 95% CI ensures that the study has sufficient statistical power to identify significant differences. The effect size of 1.1 is selected in accordance with conventional practice in comparable research settings. To achieve the desired sample size of 50 patients for the study, the patients were divided into two groups. Group A, consisting of 25 patients, received resistance and aerobic exercises. On the other hand, group B, also consisting of 25 patients was instructed to continue normal daily activities ) such as waking up, dressing, going to bed, etc.) without participating in any therapeutic exercise program.
Prior to being enrolled in the study, all participants were required to sign a consent form. The consent form was drafted in a fluent format, incorporating both English and Arabic languages, to ensure clear comprehension by all participants. The form contained all the study specifics. It also informed all participants that the findings may be utilized in publications. Each participant could withdraw at any stage. The researcher assumed full responsibility for safeguarding the safety of the participants and maintaining the confidentiality of their personal information. The experimental project received approval from Palestine Ahliya University and Augusta Victoria Hospital.
The data were collected using the following tools:
Brief fatigue inventory (BFI): The BFI is used to rapidly assess the severity and impact of fatigue, including cancer-related fatigue (CRF). A growing focus on CRF has emphasized the need for sensitive tools to evaluate this frequently reported symptom. The BFI’s six interference items correlate with standard quality-of-life measures, allowing for a quick determination of the severity and impact of fatigue. Patients assessed their level of fatigue using a numerical scale ranging from zero (indicating no fatigue) to ten (representing the most extreme fatigue imaginable). This scale enabled the classification of fatigue into mild, moderate, or severe levels [8].
Functional independence measure (FIM): This instrument assesses quality of life by rating independence in 18 activities of daily living (with scores ranging from 0 to 7; the higher the score, the better the condition). The FIM includes 13 motor items, with a total score range of 13-91, and 5 cognitive items, with a total score range of 5-35. These can be categorized into a “motor” subscale and a “cognitive” subscale [9]. The FIM was created to assess impairment across a range of demographics and is not diagnosis-specific. It measures independence for self-care, such as sphincter control, transfers, movement, communication, and social cognition. This instrument evaluates a person’s functional state based on their degree of support, ranging from complete independence to complete assistance. It assesses a patient’s degree of impairment and any changes in their condition as a result of rehabilitation or medical intervention [10].
Timed up and go (TUG) test: This is a simple test that can be performed anywhere and consists of a patient getting up from a chair, transitioning from a sitting to a bipedal position, walking three meters, turning, returning, and sitting back in the chair again. The variable measured is the total time taken to complete the test, with the score assigned in seconds being correlated with the risk of falls. Some advantages of the TUG test include its simplicity in application and its short duration. In addition, it requires minimal equipment and allows individuals with functional impairments to perform the test [11]. The TUG test is used to assess mobility, balance, and locomotor performance. Mobility is assessed based on the time taken to complete the test: Less than 10 seconds indicates normal mobility; less than 20 seconds indicates good mobility (the individual can walk outside alone and does not require a walking aid); and less than 30 seconds indicates walking and balance problems (the individual cannot walk outside alone and requires a walking aid) [11].
The data analysis used a range of software technologies. The analysis of differences between groups was conducted using SPSS software, version 24.0 (IBM Corp., Armonk, NY, USA), which offered insights via both descriptive and inferential statistics. To ensure the robustness of the study’s design and findings, statistical power and effect sizes were calculated using G*Power software, version 3.1.9.4 (Universität Düsseldorf, Germany, 2014). Data were organized and processed using Microsoft Excel software, version 2016 (Microsoft Corporation, Redmond, WA, USA).
An analysis of the sample’s characteristics was conducted using descriptive and frequency statistics. Comparisons of demographic data across groups were conducted using the independent samples t-test, and effect sizes were computed using G*Power software, version 3.1.9.4. In accordance with Cohen’s criteria, the strength of relationships was determined using small, medium, and large effect sizes. Furthermore, the treatment groups were compared using independent t-tests (Figure 7).
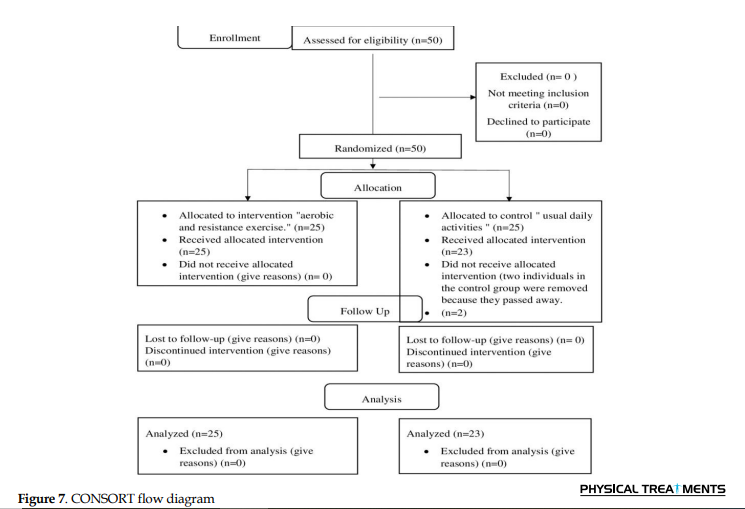
Results
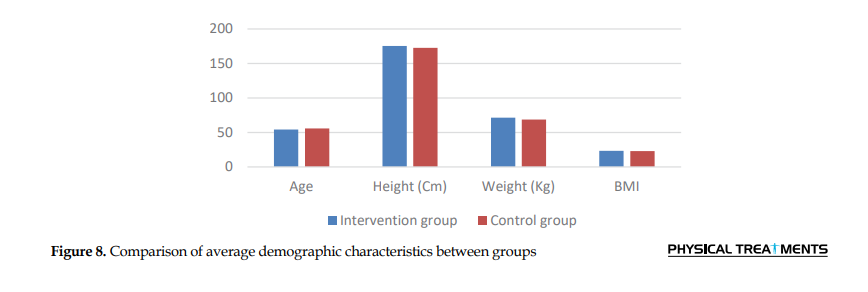
The study included 50 participants allocated into two groups: Group A and group B. The mean weight exceeded 68.7 kg, while the mean height was 175.3 cm. The mean BMI was 23.4 kg/m2 for group A and 22.9 kg/m2 for group B. The Mann-Whitney U test indicated no significant difference between the two groups, with P>0.05 for both groups (Table 1 and Figure 8).
.PNG)
The study aimed to evaluate the effects of aerobic and resistance activities on cancer patients’ fatigue, functional independence, and mobility, emphasizing beneficial outcomes.
The results shown in Table 2 and Figure 9 show that the exercise intervention produced significant improvements in all areas. The results of BFI showed that people with cancer had significantly lower levels of fatigue.
.PNG)
The mean scores fell from 67.3 to 50.8 (d=2.9, P=0.002). The considerable effect size of 2.9 suggests that the exercise intervention had a significant impact on fatigue levels. According to the study, physical exercise interventions had a significant impact on psychological well-being, as evidenced by potential changes in a variety of psychological characteristics, such as walking ability, mood, daily tasks (such as housekeeping), social interactions, and life satisfaction.
The FIM scores revealed a significant improvement in functional independence in the areas of mobility, communication, and self-care. The mean score increased from 72 before the intervention to 87 after it, showing a substantial change (P<0.001, d=-2.7). Despite the negative sign, the effect size of -2.7 shows a significant influence and highlights the noteworthy improvements in functional autonomy observed after the exercise program.
Significant improvements in mobility were also noted in the study, as evidenced by a decrease in TUG test averages from 22.08 seconds before the intervention to 15.9 seconds after it (P<0.001, d=3.2). The mobility of cancer patients was significantly improved by aerobic and strengthening exercise sessions, as evidenced by the reported effect size of 3.2. This result likewise shows a significant improvement in TUG performance on the test after the exercise intervention. According to the findings, physical activity has a good impact on a variety of cancer patients, resulting in higher activity levels and reduced fatigue. The results underscore the importance of incorporating customized physical activity programs into cancer treatment plans to improve patient outcomes. This is accomplished by showing how aerobic and resistance exercises can improve psychological health, functional independence, and mobility in cancer patients.
The results displayed in Table 3 indicate that the evaluated outcomes did not change significantly in the control group, which did not receive any therapy and maintained the usual daily activities. The BFI was utilized to evaluate fatigue. The control group’s mean BFI scores increased moderately after the intervention, from 55 to 56 (P=0.004, d=0.68). Despite a statistically significant increase in BFI scores, the results deviate from the expected enhancement direction, suggesting that the control condition may not have had any effect at all or may have had a negative impact on these aspects of physical and psychological well-being.
.PNG)
According to the FIM, there was no notable enhancement in functional independence when comparing the scores before and after the intervention (P=0.591, d=-0.11). The effect size of -0.11 indicates that there was no statistically meaningful change in functional independence after the intervention period.
Furthermore, there were no significant differences in the mobility outcomes of the TUG test prior to and following the intervention (P=0.426, d=0.17). The participants in the control group did not show any significant changes in their mobility outcomes, as evidenced by the effect size of 0.17, indicating a modest impact.
The Mann-Whitney U test was employed to examine the impact of aerobic and strengthening exercises on different cancer patients. The study primarily aimed to evaluate the alterations in the scores of the BFI, FIM, and TUG test resulting from these exercises. The investigation uncovered significant discrepancies between the two groups, in relation to all three metrics. The intervention group exhibited a substantial decrease in post-intervention fatigue levels, as indicated by the significant mean difference of -16.52 compared to the control group’s mean difference of 1.5 on the BFI. This conclusion is substantiated by a substantial effect size of 3.2.
Similarly, the scores of the FIM showed a statistically significant difference in mean scores (interventions group: 15.1, control group: -0.65). The intervention group exhibited significant improvements in functional independence after the exercise intervention, as evidenced by the substantial effect size of 2.7. Furthermore, the findings of the TUG test demonstrated a substantial average difference between the intervention group (-6.1) and the control group (0.17), suggesting enhanced mobility and agility in the intervention group following the intervention.
In summary, this study reveals that physical exercise has a positive effect on cancer patients by reducing fatigue, enhancing independent functioning, and improving mobility. These results emphasize the need to incorporate physical activity into cancer care to improve overall health and well-being, as well as the quality of life for individuals diagnosed with cancer.
Discussion
Physical activity is essential in oncology treatment, mitigating undesirable consequences, such as CRF and muscular atrophy. It improves quality of life, alleviates psychological suffering, and promotes oncological prognoses, including decreased recurrence rates and improved survival outcomes. Continuous engagement is correlated with enhanced results. Quantitative analysis revealed a statistically significant reduction in fatigue levels, as indicated by a decrease in the mean BFI scores from 67.3 to 50.8 (P=0.002, d=2.9). The FIM scores demonstrated a considerable increase from 72 to 87 (P<0.001, d=-2.7), underscoring a significant enhancement in functional autonomy. Mobility improvements were further corroborated by the TUG test, with mean completion times reduced from 22.08 seconds to 15.9 seconds (P<0.001, d=3.2). In contrast, the control group, which did not undergo any intervention, exhibited minimal changes, with a slight increase in fatigue as shown by the BFI scores (from 55 to 56, P=0.004, d=0.68) but no significant alterations in FIM scores (P=0.591, d=-0.11) or TUG performance (P=0.426, d=0.17).
The research underscores the significance of physical activity in enhancing patient outcomes and diminishing the risk of certain cancer types. It also indicates that physical activity can enhance tiredness reduction, functional autonomy, and performance in activities of daily living, beyond the advantages of routine daily activities alone.
Liu et al.’s study aimed to identify exercises that reduce CRF in breast cancer patients. They found that aerobic, resistance and combined exercise therapies were effective in reducing fatigue [12]. Combining aerobic and resistance exercises significantly improved fatigue levels, resulting in decreased fatigue and improved functional independence in cancer patients.
Mavropalias et al. examined the effects of home-based exercise therapy on fatigue in breast cancer patients receiving radiation therapy. The study revealed that individuals actively engaged in the exercise program exhibited notable enhancements in fatigue levels relative to the control group. Those in the control group received no treatment and maintained their usual daily activities; the results deviated from the expected enhancement direction, suggesting that the control condition may not have had any effect at all or may have had a negative impact on these aspects of physical and psychological well-being. This indicates that integrating home-based exercise into treatment regimens might successfully alleviate tiredness symptoms and enhance overall well-being in cancer patients [13].
McGrorry et al. examined the impact of physical activity on sleep disturbances and CRF in breast cancer survivors. Consistent physical activity enhanced sleep quality and reduced fatigue levels. The research indicated that incorporating exercise into treatment regimens may alleviate sleep disturbances and exhaustion associated with cancer, consistent with the current study [14].
Wang et al. performed a meta-analysis on exercise therapies for cancer patients to evaluate their effectiveness in alleviating CRF and enhancing quality of life. The findings indicated that exercise therapy markedly alleviated fatigue symptoms and enhanced overall well-being. The study highlights the significance of including physical activity in comprehensive cancer care protocols, with home exercise yielding comparable outcomes [15].
Kuehn et al. found that physical activity programs can significantly alleviate CRF in children, adolescents, and young adults. Regular participation in physical activity decreased tiredness and enhanced the quality of life in cancer patients [16]. Incorporating physical activity programs into holistic treatment regimens can mitigate the impact of CRF.
Plinsinga et al. discovered that physical activity can diminish the risk of cancer-related lymphedema (CRL) in individuals with cancer. The research indicated that workout cohorts with five or more lymph nodes exhibited a diminished chance of developing CRL. Exercise enhanced upper-body functionality, strength, lower-body strength, tiredness levels, and general quality of life [17]. The results support the endorsement of aerobic and resistance training for cancer patients.
Deminice et al. examined the significance of physical activity in cancer treatment in Brazil. It emphasized the advantages of consistent physical activity, such as enhanced quality of life, reduced fatigue, and better therapeutic outcomes. Aerobic and resistance activities are advised for cardiovascular health and the maintenance of muscle strength. Exercise enhances mental and emotional well-being by addressing psychosocial factors and alleviating anxiety, depression, and stress during cancer treatment. Healthcare professionals play a crucial role in providing tailored fitness programs that cater to individual patient needs and preferences. Healthcare practitioners in Brazil should prioritize the implementation of customized exercise regimens to enhance overall outcomes and improve patients’ quality of life [18]. The outcomes of this study align well with ours, as the aerobic and resistance activities conducted by the cancer patients resulted in a decrease in their degree of fatigue and an improvement in quality of life.
Jung et al. examined the effects of structured exercise therapy on CRF in pediatric patients undergoing stem cell transplantation. The study utilized a RCT design, assigning patients to either an exercise intervention group or a control group. The findings indicated that structured exercise therapy markedly diminished fatigue levels and enhanced functional results, implying its potential to facilitate recovery [19]. According to our research, cancer patients who exercise have less fatigue and decreased disability. Our research examined the effects of physical activity on different cancer patient populations, emphasizing its efficacy in alleviating fatigue, boosting quality of life, and improving balance.
Conclusion
Exercise enhances the quality of life, physical activity, and strength in cancer patients, alleviating weariness. A multidisciplinary team must evaluate patients’ physiological conditions and customize therapies according to the etiology of weariness. Integrating physical activity into cancer treatment improves overall health and quality of life.
Limitations of study
The study encountered constraints owing to restricted public access to patient data, insufficient enrollment of various cancer patients, inconsistent adherence to exercise, and the absence of long-term follow-up after eight weeks of therapy. Furthermore, certain patients were discharged to their homes, leading to ongoing communication and the continuation of home visit sessions. The study also lacked longitudinal follow-up and demonstrated heterogeneity among cancer patients. Strategies can be implemented to mitigate these restrictions in future studies, such as encouraging cancer patients to attend physical therapy centers after their hospital treatment period to facilitate the process of reaching patients and achieving better results with high accuracy. Regarding commitment to exercises, we can educate caregivers so they can remind patients to perform them.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Palestine Ahliya University, Bethlehem, Palestine (Code: CAMS/PTBR/3/132/2024) and the Universal Trial Number (Code: U1111-1311-2461). All procedures were carried out in compliance with the regulations of the Helsinki Declaration. The 2008 Helsinki Declaration was adhered to, and patients provided their informed consent prior to any data collection.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the patients who participated in the study.
References
Full-Text: (22 Views)
Introduction
Cancer is a disease marked by the unregulated growth of altered cells resulting from natural selection. It ranks as the second foremost cause of mortality worldwide, with 19.3 million new cases documented in 2020 and 10 million deaths. By 2050, there will be 35 million cancer cases, while the population of cancer survivors will rise to 20.6 million. Conventional therapies, such as surgery, chemotherapy, and radiotherapy yield limited beneficial results, necessitating the investigation of novel treatments. Potential side effects may include nausea, weariness, and diminished capacity for everyday activities [1-3].
Cancer-related tiredness is a chronic physical, emotional, or cognitive depletion in cancer patients that restricts normal functioning. Exercise is advised for enhancing health-related quality of life; however, a 2023 systematic review yielded inconclusive findings, hindering the formulation of evidence-based guidelines for the incorporation of exercise in cancer patients [4-6].
Research about the effects of physical exercise on cancer patients is expanding; nevertheless, there is a deficiency of studies examining activity and fatigue levels across various cancer types. Comprehending these distinctions is essential for developing tailored exercises and improving cancer therapy. Through the analysis of activity and exhaustion levels, researchers can discern trends and variances, allowing healthcare professionals to develop customized workout programs [7].
Research on the impact of physical exercise on cancer patients’ activity and fatigue levels can help understand the mechanisms involved. However, there is a lack of comprehensive studies on this topic. To address this, future studies should span different cancer populations and use longitudinal studies with larger sample sizes. This will facilitate the creation of tailored exercise programs and improve cancer care techniques. This study aimed to find out how physical exercise affects daily activities and fatigue levels in different types of cancer including breast, lung, and prostate cancer. Patients must be able to perform physical activities to be included in this study.
Materials and Methods
A randomized controlled trial (RCT) was conducted to investigate the impact of physical exercise on various categories of cancer patients from February to April 2024.
The total number of participants was 50 patients, who were randomly assigned to the intervention group (A) consisting of 25 patients, and the control group (B) consisting of 25 patients using random assignment. This sample size was chosen because it matched the inclusion and exclusion criteria. The patients were then divided according to the type of cancer into two groups equally, ensuring that each group included all types of cancer to enhance the accuracy of the study. Patients who were currently receiving or had recently completed treatment for various forms of cancer, such as breast, lung, or prostate cancer, were included. The cohort comprised 20 breast cancer patients, 15 lung cancer patients, and 15 prostate cancer patients, with patients diagnosed with stage II and III disease by oncologists at Augusta Victoria Hospital in Jerusalem.
A study was conducted with hospitalized cancer patients aged 18-65 who were physically capable of conducting activities and willing to participate. Exclusion criteria included recent surgical procedures, amputations, dependence on oxygen, and cognitive deficits.
Patients were randomly assigned to the two groups. A separate researcher, using a computer software program, created a randomization schedule to ensure fair allocation. This schedule generated a random sequence for assigning patients to each group. Each patient was assigned randomly to one group. The intervention group (A) participated in aerobic exercises such as walking and stationary cycling, as well as resistance exercises (20 minutes) including wall slides/partial squats, which were repeated 10-12 times with a short rest period; seated leg presses; shoulder pulls using a rubber band; and bicep curls, which were each repeated 8-10 times. The control group (B) was advised to maintain the usual daily activities and not participate in any planned exercise program (Figures 1, 2, 3, 4, 5 and 6).
Cancer is a disease marked by the unregulated growth of altered cells resulting from natural selection. It ranks as the second foremost cause of mortality worldwide, with 19.3 million new cases documented in 2020 and 10 million deaths. By 2050, there will be 35 million cancer cases, while the population of cancer survivors will rise to 20.6 million. Conventional therapies, such as surgery, chemotherapy, and radiotherapy yield limited beneficial results, necessitating the investigation of novel treatments. Potential side effects may include nausea, weariness, and diminished capacity for everyday activities [1-3].
Cancer-related tiredness is a chronic physical, emotional, or cognitive depletion in cancer patients that restricts normal functioning. Exercise is advised for enhancing health-related quality of life; however, a 2023 systematic review yielded inconclusive findings, hindering the formulation of evidence-based guidelines for the incorporation of exercise in cancer patients [4-6].
Research about the effects of physical exercise on cancer patients is expanding; nevertheless, there is a deficiency of studies examining activity and fatigue levels across various cancer types. Comprehending these distinctions is essential for developing tailored exercises and improving cancer therapy. Through the analysis of activity and exhaustion levels, researchers can discern trends and variances, allowing healthcare professionals to develop customized workout programs [7].
Research on the impact of physical exercise on cancer patients’ activity and fatigue levels can help understand the mechanisms involved. However, there is a lack of comprehensive studies on this topic. To address this, future studies should span different cancer populations and use longitudinal studies with larger sample sizes. This will facilitate the creation of tailored exercise programs and improve cancer care techniques. This study aimed to find out how physical exercise affects daily activities and fatigue levels in different types of cancer including breast, lung, and prostate cancer. Patients must be able to perform physical activities to be included in this study.
Materials and Methods
A randomized controlled trial (RCT) was conducted to investigate the impact of physical exercise on various categories of cancer patients from February to April 2024.
The total number of participants was 50 patients, who were randomly assigned to the intervention group (A) consisting of 25 patients, and the control group (B) consisting of 25 patients using random assignment. This sample size was chosen because it matched the inclusion and exclusion criteria. The patients were then divided according to the type of cancer into two groups equally, ensuring that each group included all types of cancer to enhance the accuracy of the study. Patients who were currently receiving or had recently completed treatment for various forms of cancer, such as breast, lung, or prostate cancer, were included. The cohort comprised 20 breast cancer patients, 15 lung cancer patients, and 15 prostate cancer patients, with patients diagnosed with stage II and III disease by oncologists at Augusta Victoria Hospital in Jerusalem.
A study was conducted with hospitalized cancer patients aged 18-65 who were physically capable of conducting activities and willing to participate. Exclusion criteria included recent surgical procedures, amputations, dependence on oxygen, and cognitive deficits.
Patients were randomly assigned to the two groups. A separate researcher, using a computer software program, created a randomization schedule to ensure fair allocation. This schedule generated a random sequence for assigning patients to each group. Each patient was assigned randomly to one group. The intervention group (A) participated in aerobic exercises such as walking and stationary cycling, as well as resistance exercises (20 minutes) including wall slides/partial squats, which were repeated 10-12 times with a short rest period; seated leg presses; shoulder pulls using a rubber band; and bicep curls, which were each repeated 8-10 times. The control group (B) was advised to maintain the usual daily activities and not participate in any planned exercise program (Figures 1, 2, 3, 4, 5 and 6).
.PNG)
.PNG)
.PNG)

The sample size was determined based on Cohen’s mathematical technique for sample size computation. The minimum sample size for each group was determined using the Equation 1:
.PNG)
Equation 1 includes the following variables: n denotes the necessary number of participants per group, N represents the total number of groups, Zα is the Z-value that corresponds to the desired level of statistical significance (typically set at 1.96 for a 95% confidence interval [CI]), Zβ is the Z-value that corresponds to the desired power level (typically set at 0.90 for 80% statistical power), and d represents the effect size. The selection of an 80% power level and a 95% CI ensures that the study has sufficient statistical power to identify significant differences. The effect size of 1.1 is selected in accordance with conventional practice in comparable research settings. To achieve the desired sample size of 50 patients for the study, the patients were divided into two groups. Group A, consisting of 25 patients, received resistance and aerobic exercises. On the other hand, group B, also consisting of 25 patients was instructed to continue normal daily activities ) such as waking up, dressing, going to bed, etc.) without participating in any therapeutic exercise program.
Prior to being enrolled in the study, all participants were required to sign a consent form. The consent form was drafted in a fluent format, incorporating both English and Arabic languages, to ensure clear comprehension by all participants. The form contained all the study specifics. It also informed all participants that the findings may be utilized in publications. Each participant could withdraw at any stage. The researcher assumed full responsibility for safeguarding the safety of the participants and maintaining the confidentiality of their personal information. The experimental project received approval from Palestine Ahliya University and Augusta Victoria Hospital.
The data were collected using the following tools:
Brief fatigue inventory (BFI): The BFI is used to rapidly assess the severity and impact of fatigue, including cancer-related fatigue (CRF). A growing focus on CRF has emphasized the need for sensitive tools to evaluate this frequently reported symptom. The BFI’s six interference items correlate with standard quality-of-life measures, allowing for a quick determination of the severity and impact of fatigue. Patients assessed their level of fatigue using a numerical scale ranging from zero (indicating no fatigue) to ten (representing the most extreme fatigue imaginable). This scale enabled the classification of fatigue into mild, moderate, or severe levels [8].
Functional independence measure (FIM): This instrument assesses quality of life by rating independence in 18 activities of daily living (with scores ranging from 0 to 7; the higher the score, the better the condition). The FIM includes 13 motor items, with a total score range of 13-91, and 5 cognitive items, with a total score range of 5-35. These can be categorized into a “motor” subscale and a “cognitive” subscale [9]. The FIM was created to assess impairment across a range of demographics and is not diagnosis-specific. It measures independence for self-care, such as sphincter control, transfers, movement, communication, and social cognition. This instrument evaluates a person’s functional state based on their degree of support, ranging from complete independence to complete assistance. It assesses a patient’s degree of impairment and any changes in their condition as a result of rehabilitation or medical intervention [10].
Timed up and go (TUG) test: This is a simple test that can be performed anywhere and consists of a patient getting up from a chair, transitioning from a sitting to a bipedal position, walking three meters, turning, returning, and sitting back in the chair again. The variable measured is the total time taken to complete the test, with the score assigned in seconds being correlated with the risk of falls. Some advantages of the TUG test include its simplicity in application and its short duration. In addition, it requires minimal equipment and allows individuals with functional impairments to perform the test [11]. The TUG test is used to assess mobility, balance, and locomotor performance. Mobility is assessed based on the time taken to complete the test: Less than 10 seconds indicates normal mobility; less than 20 seconds indicates good mobility (the individual can walk outside alone and does not require a walking aid); and less than 30 seconds indicates walking and balance problems (the individual cannot walk outside alone and requires a walking aid) [11].
The data analysis used a range of software technologies. The analysis of differences between groups was conducted using SPSS software, version 24.0 (IBM Corp., Armonk, NY, USA), which offered insights via both descriptive and inferential statistics. To ensure the robustness of the study’s design and findings, statistical power and effect sizes were calculated using G*Power software, version 3.1.9.4 (Universität Düsseldorf, Germany, 2014). Data were organized and processed using Microsoft Excel software, version 2016 (Microsoft Corporation, Redmond, WA, USA).
An analysis of the sample’s characteristics was conducted using descriptive and frequency statistics. Comparisons of demographic data across groups were conducted using the independent samples t-test, and effect sizes were computed using G*Power software, version 3.1.9.4. In accordance with Cohen’s criteria, the strength of relationships was determined using small, medium, and large effect sizes. Furthermore, the treatment groups were compared using independent t-tests (Figure 7).

Results
The study included 50 participants allocated into two groups: Group A and group B. The mean weight exceeded 68.7 kg, while the mean height was 175.3 cm. The mean BMI was 23.4 kg/m2 for group A and 22.9 kg/m2 for group B. The Mann-Whitney U test indicated no significant difference between the two groups, with P>0.05 for both groups (Table 1 and Figure 8).
.PNG)

The study aimed to evaluate the effects of aerobic and resistance activities on cancer patients’ fatigue, functional independence, and mobility, emphasizing beneficial outcomes.
The results shown in Table 2 and Figure 9 show that the exercise intervention produced significant improvements in all areas. The results of BFI showed that people with cancer had significantly lower levels of fatigue.
.PNG)

The mean scores fell from 67.3 to 50.8 (d=2.9, P=0.002). The considerable effect size of 2.9 suggests that the exercise intervention had a significant impact on fatigue levels. According to the study, physical exercise interventions had a significant impact on psychological well-being, as evidenced by potential changes in a variety of psychological characteristics, such as walking ability, mood, daily tasks (such as housekeeping), social interactions, and life satisfaction.
The FIM scores revealed a significant improvement in functional independence in the areas of mobility, communication, and self-care. The mean score increased from 72 before the intervention to 87 after it, showing a substantial change (P<0.001, d=-2.7). Despite the negative sign, the effect size of -2.7 shows a significant influence and highlights the noteworthy improvements in functional autonomy observed after the exercise program.
Significant improvements in mobility were also noted in the study, as evidenced by a decrease in TUG test averages from 22.08 seconds before the intervention to 15.9 seconds after it (P<0.001, d=3.2). The mobility of cancer patients was significantly improved by aerobic and strengthening exercise sessions, as evidenced by the reported effect size of 3.2. This result likewise shows a significant improvement in TUG performance on the test after the exercise intervention. According to the findings, physical activity has a good impact on a variety of cancer patients, resulting in higher activity levels and reduced fatigue. The results underscore the importance of incorporating customized physical activity programs into cancer treatment plans to improve patient outcomes. This is accomplished by showing how aerobic and resistance exercises can improve psychological health, functional independence, and mobility in cancer patients.
The results displayed in Table 3 indicate that the evaluated outcomes did not change significantly in the control group, which did not receive any therapy and maintained the usual daily activities. The BFI was utilized to evaluate fatigue. The control group’s mean BFI scores increased moderately after the intervention, from 55 to 56 (P=0.004, d=0.68). Despite a statistically significant increase in BFI scores, the results deviate from the expected enhancement direction, suggesting that the control condition may not have had any effect at all or may have had a negative impact on these aspects of physical and psychological well-being.
.PNG)
According to the FIM, there was no notable enhancement in functional independence when comparing the scores before and after the intervention (P=0.591, d=-0.11). The effect size of -0.11 indicates that there was no statistically meaningful change in functional independence after the intervention period.
Furthermore, there were no significant differences in the mobility outcomes of the TUG test prior to and following the intervention (P=0.426, d=0.17). The participants in the control group did not show any significant changes in their mobility outcomes, as evidenced by the effect size of 0.17, indicating a modest impact.
The Mann-Whitney U test was employed to examine the impact of aerobic and strengthening exercises on different cancer patients. The study primarily aimed to evaluate the alterations in the scores of the BFI, FIM, and TUG test resulting from these exercises. The investigation uncovered significant discrepancies between the two groups, in relation to all three metrics. The intervention group exhibited a substantial decrease in post-intervention fatigue levels, as indicated by the significant mean difference of -16.52 compared to the control group’s mean difference of 1.5 on the BFI. This conclusion is substantiated by a substantial effect size of 3.2.
Similarly, the scores of the FIM showed a statistically significant difference in mean scores (interventions group: 15.1, control group: -0.65). The intervention group exhibited significant improvements in functional independence after the exercise intervention, as evidenced by the substantial effect size of 2.7. Furthermore, the findings of the TUG test demonstrated a substantial average difference between the intervention group (-6.1) and the control group (0.17), suggesting enhanced mobility and agility in the intervention group following the intervention.
In summary, this study reveals that physical exercise has a positive effect on cancer patients by reducing fatigue, enhancing independent functioning, and improving mobility. These results emphasize the need to incorporate physical activity into cancer care to improve overall health and well-being, as well as the quality of life for individuals diagnosed with cancer.
Discussion
Physical activity is essential in oncology treatment, mitigating undesirable consequences, such as CRF and muscular atrophy. It improves quality of life, alleviates psychological suffering, and promotes oncological prognoses, including decreased recurrence rates and improved survival outcomes. Continuous engagement is correlated with enhanced results. Quantitative analysis revealed a statistically significant reduction in fatigue levels, as indicated by a decrease in the mean BFI scores from 67.3 to 50.8 (P=0.002, d=2.9). The FIM scores demonstrated a considerable increase from 72 to 87 (P<0.001, d=-2.7), underscoring a significant enhancement in functional autonomy. Mobility improvements were further corroborated by the TUG test, with mean completion times reduced from 22.08 seconds to 15.9 seconds (P<0.001, d=3.2). In contrast, the control group, which did not undergo any intervention, exhibited minimal changes, with a slight increase in fatigue as shown by the BFI scores (from 55 to 56, P=0.004, d=0.68) but no significant alterations in FIM scores (P=0.591, d=-0.11) or TUG performance (P=0.426, d=0.17).
The research underscores the significance of physical activity in enhancing patient outcomes and diminishing the risk of certain cancer types. It also indicates that physical activity can enhance tiredness reduction, functional autonomy, and performance in activities of daily living, beyond the advantages of routine daily activities alone.
Liu et al.’s study aimed to identify exercises that reduce CRF in breast cancer patients. They found that aerobic, resistance and combined exercise therapies were effective in reducing fatigue [12]. Combining aerobic and resistance exercises significantly improved fatigue levels, resulting in decreased fatigue and improved functional independence in cancer patients.
Mavropalias et al. examined the effects of home-based exercise therapy on fatigue in breast cancer patients receiving radiation therapy. The study revealed that individuals actively engaged in the exercise program exhibited notable enhancements in fatigue levels relative to the control group. Those in the control group received no treatment and maintained their usual daily activities; the results deviated from the expected enhancement direction, suggesting that the control condition may not have had any effect at all or may have had a negative impact on these aspects of physical and psychological well-being. This indicates that integrating home-based exercise into treatment regimens might successfully alleviate tiredness symptoms and enhance overall well-being in cancer patients [13].
McGrorry et al. examined the impact of physical activity on sleep disturbances and CRF in breast cancer survivors. Consistent physical activity enhanced sleep quality and reduced fatigue levels. The research indicated that incorporating exercise into treatment regimens may alleviate sleep disturbances and exhaustion associated with cancer, consistent with the current study [14].
Wang et al. performed a meta-analysis on exercise therapies for cancer patients to evaluate their effectiveness in alleviating CRF and enhancing quality of life. The findings indicated that exercise therapy markedly alleviated fatigue symptoms and enhanced overall well-being. The study highlights the significance of including physical activity in comprehensive cancer care protocols, with home exercise yielding comparable outcomes [15].
Kuehn et al. found that physical activity programs can significantly alleviate CRF in children, adolescents, and young adults. Regular participation in physical activity decreased tiredness and enhanced the quality of life in cancer patients [16]. Incorporating physical activity programs into holistic treatment regimens can mitigate the impact of CRF.
Plinsinga et al. discovered that physical activity can diminish the risk of cancer-related lymphedema (CRL) in individuals with cancer. The research indicated that workout cohorts with five or more lymph nodes exhibited a diminished chance of developing CRL. Exercise enhanced upper-body functionality, strength, lower-body strength, tiredness levels, and general quality of life [17]. The results support the endorsement of aerobic and resistance training for cancer patients.
Deminice et al. examined the significance of physical activity in cancer treatment in Brazil. It emphasized the advantages of consistent physical activity, such as enhanced quality of life, reduced fatigue, and better therapeutic outcomes. Aerobic and resistance activities are advised for cardiovascular health and the maintenance of muscle strength. Exercise enhances mental and emotional well-being by addressing psychosocial factors and alleviating anxiety, depression, and stress during cancer treatment. Healthcare professionals play a crucial role in providing tailored fitness programs that cater to individual patient needs and preferences. Healthcare practitioners in Brazil should prioritize the implementation of customized exercise regimens to enhance overall outcomes and improve patients’ quality of life [18]. The outcomes of this study align well with ours, as the aerobic and resistance activities conducted by the cancer patients resulted in a decrease in their degree of fatigue and an improvement in quality of life.
Jung et al. examined the effects of structured exercise therapy on CRF in pediatric patients undergoing stem cell transplantation. The study utilized a RCT design, assigning patients to either an exercise intervention group or a control group. The findings indicated that structured exercise therapy markedly diminished fatigue levels and enhanced functional results, implying its potential to facilitate recovery [19]. According to our research, cancer patients who exercise have less fatigue and decreased disability. Our research examined the effects of physical activity on different cancer patient populations, emphasizing its efficacy in alleviating fatigue, boosting quality of life, and improving balance.
Conclusion
Exercise enhances the quality of life, physical activity, and strength in cancer patients, alleviating weariness. A multidisciplinary team must evaluate patients’ physiological conditions and customize therapies according to the etiology of weariness. Integrating physical activity into cancer treatment improves overall health and quality of life.
Limitations of study
The study encountered constraints owing to restricted public access to patient data, insufficient enrollment of various cancer patients, inconsistent adherence to exercise, and the absence of long-term follow-up after eight weeks of therapy. Furthermore, certain patients were discharged to their homes, leading to ongoing communication and the continuation of home visit sessions. The study also lacked longitudinal follow-up and demonstrated heterogeneity among cancer patients. Strategies can be implemented to mitigate these restrictions in future studies, such as encouraging cancer patients to attend physical therapy centers after their hospital treatment period to facilitate the process of reaching patients and achieving better results with high accuracy. Regarding commitment to exercises, we can educate caregivers so they can remind patients to perform them.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Palestine Ahliya University, Bethlehem, Palestine (Code: CAMS/PTBR/3/132/2024) and the Universal Trial Number (Code: U1111-1311-2461). All procedures were carried out in compliance with the regulations of the Helsinki Declaration. The 2008 Helsinki Declaration was adhered to, and patients provided their informed consent prior to any data collection.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the patients who participated in the study.
References
- Bower JE, Kuhlman KR. Psychoneuroimmunology: An introduction to immune-to-brain communication and its implications for clinical psychology. Annual Review of Clinical Psychology. 2023; 19:331-59. [DOI:10.1146/annurev-clinpsy-080621-045153] [PMID]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2021; 71(3):209-49. [DOI:10.3322/caac.21660] [PMID]
- Krasteva N, Georgieva M. Promising therapeutic strategies for colorectal cancer treatment based on nanomaterials. Pharmaceutics. 2022; 14(6):1213. [DOI:10.3390/pharmaceutics14061213] [PMID]
- Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Medicine and Science in Sports and Exercise. 2019; 51(11):2375-90. [DOI:10.1249/MSS.0000000000002116] [PMID]
- Lobefaro R, Rota S, Porcu L, Brunelli C, Alfieri S, Zito E, et al. Cancer-related fatigue and depression: A monocentric, prospective, cross-sectional study in advanced solid tumors. ESMO Open. 2022; 7(2):100457. [DOI:10.1016/j.esmoop.2022.100457] [PMID]
- Spanoudaki M, Giaginis C, Karafyllaki D, Papadopoulos K, Solovos E, Antasouras G, et al. Exercise as a promising agent against cancer: evaluating its anti-cancer molecular mechanisms. Cancers. 2023; 15(21):5135. [DOI:10.3390/cancers15215135] [PMID]
- Thomsen SN, Lahart IM, Thomsen LM, Fridh MK, Larsen A, Mau-Sørensen M, et al. Harms of exercise training in patients with cancer undergoing systemic treatment: a systematic review and meta-analysis of published and unpublished controlled trials. EClinicalMedicine. 2023; 59:101937. [DOI:10.1016/j.eclinm.2023.101937] [PMID]
- Frikkel J, Götte M, Beckmann M, Kasper S, Hense J, Teufel M, et al. Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced Cancer patients. BMC palliative Care. 2020; 19(1):43. [DOI:10.1186/s12904-020-00542-z] [PMID]
- Fisher MI, Cohn JC, Harrington SE, Lee JQ, Malone D. Correction to: Screening and assessment of cancer-related fatigue: A clinical practice guideline for health care providers. Physical Therapy. 2024; 104(9):pzae126. [DOI:10.1093/ptj/pzae126] [PMID]
- Maki Y, Morita A, Makizako H. Association between the cognitive-related behavioral assessment severity stage and activities of daily living required for discharge to home in patients with stroke: A cross-sectional study. International Journal of Environmental Research and Public Health. 2023; 20(4):3005. [DOI:10.3390/ijerph20043005] [PMID]
- Chien SY, Wong AM, Wu CY, Beckman SL. Interactive electronic pegboard for enhancing manual dexterity and cognitive abilities: Instrument usability study. JMIR Human Factors. 2024; 11:e56357. [DOI:10.2196/56357] [PMID]
- Liu YC, Hung TT, Konara Mudiyanselage SP, Wang CJ, Lin MF. Beneficial exercises for cancer-related fatigue among women with breast cancer: A systematic review and network meta-analysis. Cancers. 2022; 15(1):151. [DOI:10.3390/cancers15010151] [PMID]
- Mavropalias G, Cormie P, Peddle-McIntyre CJ, Galvão DA, Taaffe DR, Schofield C, et al. The effects of home-based exercise therapy for breast cancer-related fatigue induced by radical radiotherapy. Breast Cancer. 2023; 30(1):139-50. [DOI:10.1007/s12282-022-01408-3] [PMID]
- McGrorry AR, Paterson A, Peddie N. The effects of exercise on sleep disturbances and cancer‐related fatigue for female breast cancer survivors receiving adjuvant hormone therapy: A systematic review. Lifestyle Medicine. 2023; 4(4):e292. [DOI:10.1002/lim2.92]
- Wang Y, Yang L, Lin G, Huang B, Sheng X, Wang L, et al. The efficacy of progressive muscle relaxation training on cancer-related fatigue and quality of life in patients with cancer: A systematic review and meta-analysis of randomized controlled studies. International Journal of Nursing Studies. 2024; 152:104694. [DOI:10.1016/j.ijnurstu.2024.104694] [PMID]
- Kuehn M, Wypyrsczyk L, Stoessel S, Neu MA, Ploch L, Dreismickenbecker E, et al. Physical activity as a treatment for cancer-related fatigue in children, adolescents and young adults: A systematic review. Children. 2023; 10(3):572. [DOI:10.3390/children10030572] [PMID]
- Plinsinga ML, Singh B, Rose GL, Clifford B, Bailey TG, Spence RR, et al. The effect of exercise on pain in people with cancer: A systematic review with meta-analysis. Sports Medicine. 2023; 53(9):1737-52. [DOI:10.1007/s40279-023-01862-9] [PMID]
- Deminice R, Rezende LFM, Rosa DD, Cangussu R, Garcia LMT, Riera R, et al. Physical activity recommendations for cancer prevention and control: A Brazilian consortium. Brazilian Journal of Oncology. 2022; 18:e-20220311. [DOI:10.5935/2526-8732.20220311]
- Jung MW, Wallek S, Senn-Malashonak A, Schubert P, Siegler K, Rosenhagen A, et al. Effects of a structured exercise therapy on cancer-related fatigue during and after paediatric stem cell transplantation: A randomized controlled trial. Physiotherapy Quarterly. 2021; 29(3):76-85. [DOI:10.5114/pq.2021.107847]
Type of Study: Research |
Subject:
General
Received: 2024/12/5 | Accepted: 2025/01/21 | Published: 2025/07/13
Received: 2024/12/5 | Accepted: 2025/01/21 | Published: 2025/07/13
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







.PNG)
