Thu, Dec 11, 2025
Volume 15, Issue 2 (Spring 2025)
PTJ 2025, 15(2): 165-174 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
S D, A S A, Suganthirababu P, Srinivasan V, Kumar P, Jayaraj V, et al . Effectiveness of Cognitive Behavioral Therapy and Aerobic Exercise in Parkinson Disease Dementia: A Pilot Study. PTJ 2025; 15 (2) :165-174
URL: http://ptj.uswr.ac.ir/article-1-689-en.html
URL: http://ptj.uswr.ac.ir/article-1-689-en.html
Dhanusia S *1 

 , Shareen Akbar A1
, Shareen Akbar A1 

 , Prathap Suganthirababu1
, Prathap Suganthirababu1 

 , Vignesh Srinivasan1
, Vignesh Srinivasan1 

 , Priyadharshini Kumar1
, Priyadharshini Kumar1 

 , Vanitha Jayaraj1
, Vanitha Jayaraj1 

 , Suriya Vishnuraman2
, Suriya Vishnuraman2 

 , Yamini Umasankar1
, Yamini Umasankar1 

 , Poovarasan Murugaiyan1
, Poovarasan Murugaiyan1 




 , Shareen Akbar A1
, Shareen Akbar A1 

 , Prathap Suganthirababu1
, Prathap Suganthirababu1 

 , Vignesh Srinivasan1
, Vignesh Srinivasan1 

 , Priyadharshini Kumar1
, Priyadharshini Kumar1 

 , Vanitha Jayaraj1
, Vanitha Jayaraj1 

 , Suriya Vishnuraman2
, Suriya Vishnuraman2 

 , Yamini Umasankar1
, Yamini Umasankar1 

 , Poovarasan Murugaiyan1
, Poovarasan Murugaiyan1 


1- Department of Neurosciences, Saveetha College of Physiotherapy, Saveetha Institute of Medical and Technical Science, Chennai, India.
2- Department of Community, Geriatrics and Palliative Care, Saveetha College of Physiotherapy, Saveetha Institute of Medical and Technical Science, Chennai, India.
2- Department of Community, Geriatrics and Palliative Care, Saveetha College of Physiotherapy, Saveetha Institute of Medical and Technical Science, Chennai, India.
Keywords: Parkinson disease, Memory, Attention, Dementia, Cognitive behavioral therapy, Aerobic exercise
Full-Text [PDF 490 kb]
(624 Downloads)
| Abstract (HTML) (2595 Views)
Full-Text: (385 Views)
Introduction
Parkinson disease (PD) is the second most prevalent neurodegenerative disorder after Alzheimer disease. PD affected people of all races and ethnicities worldwide [1]. Globally, Parkinson disease affects approximately 1.51 individuals per 1000 people across all ages [2]; it is estimated that the prevalence of Parkinson disease dementia (PDD) is around 24%–31% [3].
Motor symptoms such as bradykinesia, stiffness, resting tremors, and irregularities in posture and gait were the first signs of PD [4]. Within the discipline of neuroscience, there was increased convergence in research on non-motor abnormalities like autonomic dysfunction, cognitive impairment, and psychiatric symptoms [5]. The features of cognitive impairment in PD can vary considerably in terms of which cognitive domains were affected, as well as in terms of the time of start and rate of development, much like the characteristics of motor symptoms [6]. The cognitive profile in PD had four problems of cognitive impairment: Attention and frontal executive function, memory, visuospatial skills, and language [7].
The cognitive impairment risk factor in PD was age factor (male>female), age of onset (>65), family history, psychiatric features, medication use (deprenyl, amantadine, agonist, anticholinergics, and cumulative exposure to levodopa), which resulted in increased mortality [8]. The investigation of cognitive decline in PD includes cerebrovascular fluid biomarkers, which showed aggregated α-synuclein, increased CSF tau protein, and reduced amount of β‐amyloid 1–42 and neuroimaging techniques such as magnetic resonance imaging and Positron emission tomography to detect brain pathology [9].
Dementia is a clinical syndrome characterized by acquired loss of emotional and cognitive abilities and increased mortality compared to non-dementia PD severe enough to interfere with daily functioning and quality of life [10]. Dementia affects 10% to 80% of individuals with PD at age 60, according to community-based studies, while the prevalence was only around 30% [11]. Up to 36% of PD sufferers with a recent diagnosis show cognitive impairment. The impact of dementia on the patient, the caregiver, and the wider community was significant [6]. Dementia and cognitive decline have an important clinical impact on PD [9]. Numerous research studies have conclusively shown their adverse impact on patient quality of life and the burden on care receivers and caregivers, and specialized therapeutic approaches are needed [12].
Attention is the process of filtering information related to external and internal stimuli modulated by the prefrontal cortex, which can be classified into simple and complex attention [13]. Memory was not a single, cohesive concept. Instead, various memory systems in different forms are supported by multiple neural systems of brain areas that could save data and access it later [14]. It is an essential physiological function that depends on medial temporal structures and is required for survival [13]. Memory is classified into emotional memory, implicit memory, and explicit memory. The amygdala, hippocampus, and other brain regions are necessary for emotional and explicit memory. The basal ganglia, motor cortex, and cerebellum support implicit memory [7].
Cognitive behavioral therapy (CBT) involves a set of clinical interventions designed to improve non-motor cognitive impairment, such as attention flexibility, memory, and behavior changes. CBT is an efficient, time-limited, cost-effective, and brief treatment. CBT has been designed to treat specific symptoms and behavioral patterns of the patient in as few as 10-20 sessions through assisted therapy programs, group format, self-help materials, and bibliography [15]. The program for CBT enhances the training of the brain’s prefrontal executive functions, working memory, and attention [16].
Aerobic exercise (AE) increases fitness, helps maintain good cognitive function, and benefits older people [17]. Additionally, physical activity has advantageous impacts on physiological functions, including glucose management and cardiovascular health, which increases the chance of Alzheimer disease and cognitive decline when impaired [18]. Aerobic exercise impacts cognitive functions such as working memory, inhibition, multitasking, planning, and selective attention, and these effects could be more prominent in older women than in older males. AE improves global cognitive functions such as memory and attention in mild cognitive impairment patients [19, 20].
CBT targets cognitive restructuring and emotional regulation, while AE enhances physical health and neuroplasticity. Comparing these interventions is crucial to determine whether one is superior or combining both yields synergistic benefits for mental health and cognitive functioning. This research will tip off clinicians’ evidence-based treatment decisions for optimal patient outcomes.
The research aimed to determine the effectiveness of CBT and AE on memory and attention in patients with dementia-related PD.
2. Materials and Methods
Study design and participants
A pilot study was conducted on PD patients with dementia in a private setting. The study was explained to all 30 participants, and their informed consent was obtained. A total of 30 participants were randomly assigned to groups A (n=15) and B (n=15) using a sealed envelope method. Eligible participants completed a pre-test evaluation, and values were recorded. For 6 weeks, group A received 30 minutes of cognitive behavioral therapy every day, while group B engaged in daily aerobic exercise for the same duration. A post-test outcome measure was recorded following the 6-week intervention.
Inclusion criteria
The inclusion criteria included both genders (male and female), above 60 years of age, at least 5 years of having PDD, mild to moderate cognitive impairment such as memory and attention with a mini-mental state examination (MMSE) score of less than 23, and Parkinson disease-cognitive rating scale (PD-CRS) less than 101, and agreement to cooperate with the treatment and follow up.
Exclusion criteria
The exclusion criteria included age below 60, diagnosis of dementia due to causes other than PD, MMSE score below 10, severe psychiatric disorders, participants with severe motor dysfunction, having cardiovascular disease, and not interested in participating in the study. Outcome measures were the PD-CRS and MMSE.
Assessment measures
Parkinson disease-cognitive rating scale (PD-CRS)
The PD-CRS is a reliable screening tool for identifying PD-related cognitive impairment. It consists of 2 cortical items, clock copying (scores 0–10) and confrontation naming (scores 0–20), and 7 frontal-subcortical items: Working memory (scores 0–10), sustained attention, clock drawing, alternating and action verbal fluencies (scores 0–20 and 0–30, respectively), and immediate and delayed free-recall verbal memory (scores 0–12 for both). All item scores are added up to produce a PD-CRS score (0–134), which is split into cortical (0–30) and frontal-subcortical (0–104) scores. PD-CRS has demonstrated exceptional clinical utility, high test-retest reliability (ICC>0.90), high inter-rater reliability (ICC>0.90), and strong internal consistency (α=0.94) [21].
Mini-mental state examination (MMSE)
The MMSE consists of 11 items that assess orientation, registration, attention or calculation (spelling or serial sevens), recall, naming, repetition, comprehension (written and verbal), writing, and construction. The total score ranges from 0 to 30, with a 25 or higher representing normal cognitive status. The scores are categorized as follows: Severe (0–17), mild (18–23), and normal (24–30).
The MMSE-I (modified MMSE) has demonstrated exceptional diagnostic accuracy, with a sensitivity of 99.0%-100.0%, specificity of 98.5%-97.0%, and area under the curve of 1.0/1.0. The internal consistency reliability of the MMSE-I was high (the Cronbach α=0.70) [22].
Study procedure
Eligible participants completed a pre-test evaluation, and values were recorded. Group A underwent 30 minutes a day of cognitive behavioral therapy for 6 weeks, and Group B underwent daily aerobic exercise for 6 weeks. A post-test treatment outcome measure was recorded following a 6-week intervention.
Memory and attention adaptation training
Memory and attention adaptation training (MAAT) was a brief CBT aimed at improving cognitive failure, such as memory and attention in daily living, and enhancing the overall quality of life of the patient. MAAT was given through offline mode in the private setting daily for 6 weeks with a session of 30 minutes. MAAT consists of four steps: Education, self-awareness and monitoring, self-regulation, and compensatory strategies.
The first step, education, begins by educating patients about common memory and attention challenges associated with PD and dementia. It highlights how negative thought patterns can impact daily life. It provides an interactive session where patients share specific examples of challenging tasks, such as misplacing items or forgetting appointments. Then, it introduces the concept of cognitive lapses and emphasizes how external distractions or internal stress can interfere with performance. The education step guides setting small, manageable daily goals, like remembering to take medications on time. It creates a weekly planner for tracking simple tasks and engages patients in cognitive activities such as crossword puzzles and word association exercises. The session is concluded by reflecting on the progress made in recognizing cognitive challenges.
The second step is self-awareness and monitoring. It involves practical exercises and guides patients in identifying internal and external distractions using mock scenarios, such as multitasking while organizing tasks. It teaches the importance of monitoring eating habits and documenting emotions or distractions that affect attention. In this step, templates are provided for tracking meal timings and emotional states, encouraging patients to observe their impact on focus. It assigns a take-home task where patients record two to three memory lapses or attention drifts. Finally, more challenging cognitive activities such as Sudoku or chess are introduced to enhance concentration and engagement.
The third step is self-regulation by teaching relaxation techniques, such as diaphragmatic breathing, to calm the mind encouraging patients to practice slow, deep breathing for 10 minutes daily while focusing on their breath. Then, progressive muscle relaxation is introduced. It targets specific muscle groups to reduce physical and emotional tension. The role of relaxation is discussed in alleviating stress and improving concentration. Patients will practice these techniques and reflect on their effects on emotional regulation and focus during the sessions.
The fourth step is compensatory strategies, comprising memory and attention management. Compensatory techniques are introduced to help patients manage memory and attention challenges better. Then, verbal rehearsal is taught, such as repeating steps aloud while completing tasks like preparing tea. The patients are trained using organizational tools like day planners or calendars to simplify daily routines. It works with patients to plan a mock weekly schedule, incorporating designated time slots for meals, exercise, and relaxation. Training is also provided on digital reminders, alarms, and visual cues to improve task management. Patients will practice setting up these aids on their devices during the session.
Finally, there are integration and goal achievement, consolidation, and reflection. The strategies learned over the past five weeks are reviewed and integrated in this step. Then, patients’ most effective techniques are discussed, and personalized adaptations are encouraged for future use. Group-based problem-solving activities such as collaborative puzzles or memory games are conducted to foster engagement and reinforce learned skills. Ultimately, individual and group achievements are celebrated, and patients are encouraged to maintain these practices in their daily routines.
PD-CRS and MMSE are suitable outcome measures for MAAT due to their specificity, comprehensiveness, and sensitivity to cognitive decline, standardization, brief administration, and vast establishment [23, 24].
Aerobic exercise
Aerobic exercise consists of a 6-week daily session for 30 minutes in a private setting. Aerobic exercise involves the first 5 min for warm-up and gentle stretching targeting specific muscle groups of the upper and lower extremities and 20 min of continuous cycling on a stationary bicycle with maximum speed, which refers to the highest achievable speed by an individual, typically measured in revolutions per minute (RPM) with the initial target of 50% maximum heart rate during the first week (60%-70% RPM) and then increased gradually to 75% by the sixth week (90% RPM). The maximum heart rate is calculated using the formula 220-Age, and the last 5 min is a cool-down period. If the participant has a target heart rate of <50%, alternative strategies like brisk walking and resistance exercises can be considered. The protocol’s effectiveness will be assessed through maximum oxygen uptake (VO2 max), muscle strength and endurance, and quality of life (evaluated using the 36-item short-form survey questionnaire).
Results
A sample of 30 was analyzed, data were collected from the participants using PD-CRS and MMSE, and pre-test and post-test values were noted after CBT and AE intervention. The result showed a P<0.001 and was considered more statistically significant with CBT than AE.
Group A pre-test and post-test values of MMSE (paired t-test)
CBT intervention significantly improved memory and attention in participants, as measured by MMSE (pre-test: 17.8±2.90, post-test: 27.2±2.64, P<0.001) (Table 1).

Group B pre-test and post-test values of MMSE (paired t-test)
AE intervention significantly improved memory and attention in participants, as measured by MMSE (pre-test: 17.8±2.90, post-test: 27.2±2.64, P<0.001) (Table 2).
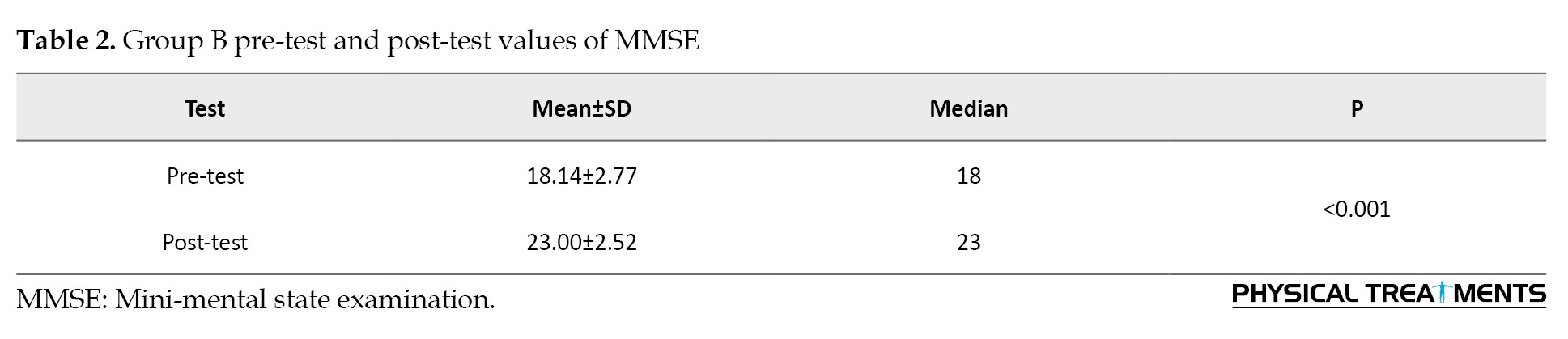
Group A and Group B post-test of MMSE (independent t-test)
Both groups showed significant improvements from the pre-test to the post-test. However, group A (CBT) had significantly greater improvements compared to group B (AE) where P<0.001. The effect size calculated is 1.72, indicating a substantial difference between groups A and B (Table 3).
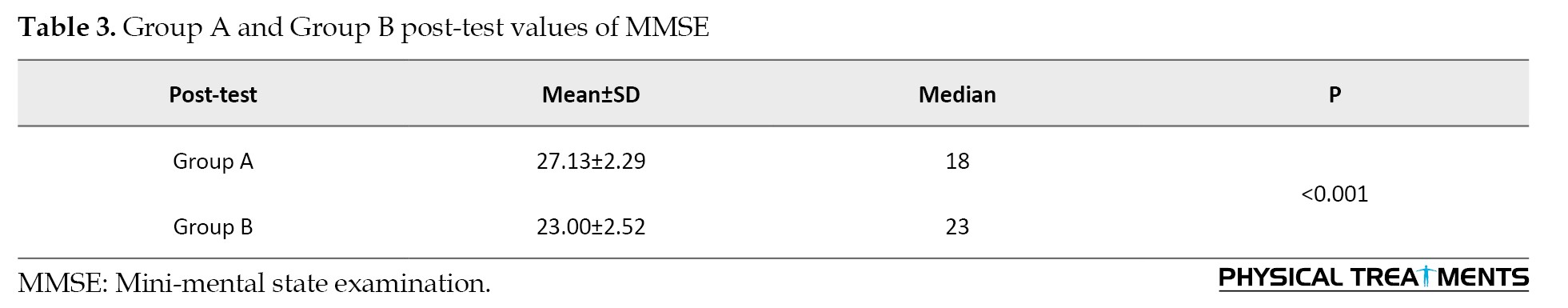
Group A pre-test and post-test values of PD-CRS (paired t-test)
CBT intervention significantly improved memory and attention in participants, as measured by PD-CRS (pre-test: 88.07±4.55, post-test: 92.07±4.95, P<0.001) (Table 4).
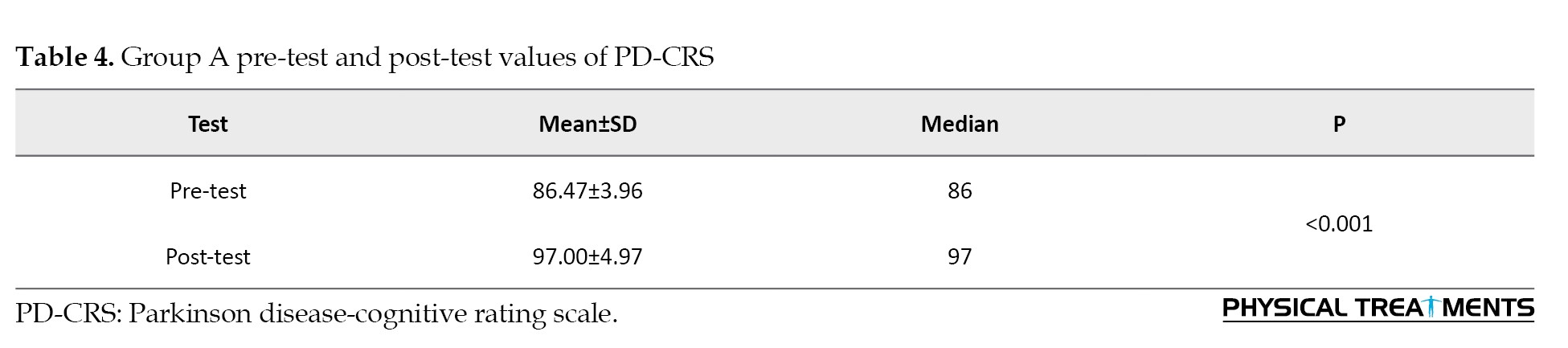
Group B pre-test and post-test values of PD-CRS (paired t-test)
AE intervention did not significantly improve memory and attention in participants, as measured by PD-CRS (pre-test: 86.47±3.83, post-test: 91.00±22.70, P=0.47) (Table 5).
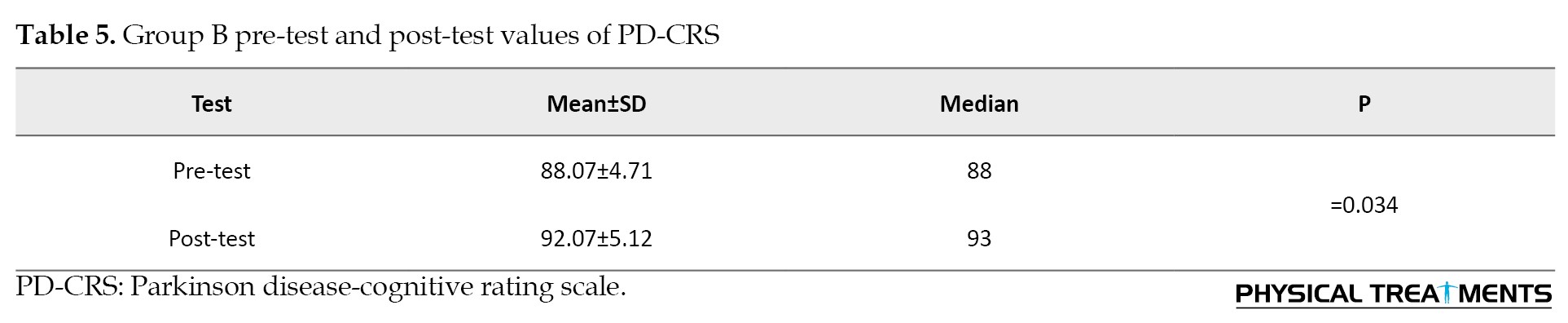
Group A and Group B post-test values of PD-CRS (independent t-test)
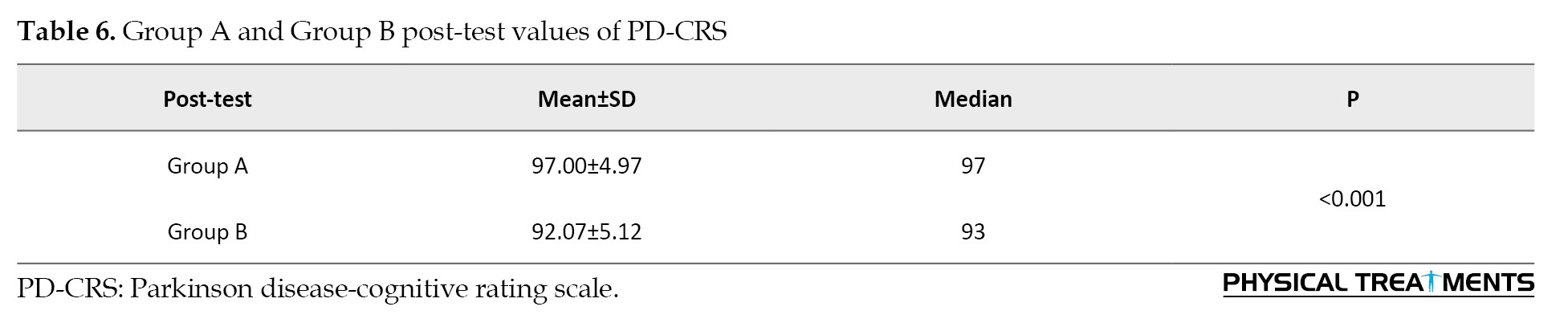
Group A significantly improved from pre-test to post-test, while group B did not. There was no significant difference between the groups in post-test scores, indicating that both interventions had similar effects on PD-CRS outcomes where P=0.93. The calculated effect size is 0.98, indicating a large effect size and a strong difference between groups A and B (Table 6).
Discussion
The present study assessed the effectiveness of cognitive behavioral therapy and aerobic exercise in memory and attention in dementia-associated PD. Generally, PD is associated with dementia, and patients have impaired cognitive function. The present study also showed improved cognitive function, and CBT was more effective and beneficial than aerobic exercise.
AE’s cognitive benefits are less consistent and generalized than CBT, and PDD patients may struggle with AE due to motor symptoms, fatigue, and balance issues. Due to this reason, CBT is considered superior to AE [25, 26].
Our findings are supported by another study indicating that CBT effectively sustains attention among children with specific learning disorders. However, this intervention does not affect working and sustained memory in children with specific learning disorders.
Previous research reveals that those with long-term breast cancer who had undergone a brief program of CBT to manage the cognitive side effects of chemotherapy report better quality of life, improved daily cognitive function, and performance on neuropsychological tests. Participants reported high levels of treatment satisfaction and assessed MAAT as beneficial for learning and implementing cognitive compensatory methods to enhance abilities to make up for memory issues [27].
According to our research, individuals with mild cognitive impairment (MCI) who engaged in AE show improvement in their overall cognitive function and memory [19]. According to recent studies, AE was used as a practice that was both cost-effective and linked to a variety of physical advantages. According to the study’s findings, some people with MCI might also improve cognitively from exercise. For executive control tasks, AE has the strongest cognitive-improving effects in women. The at-risk group’s cognitive function could be enhanced with just 6 months of behavioral therapy using periodic increases in heart rate without the expense or side effects of most pharmaceutical treatments [20].
Recent studies show that AE could help older MCI patients with general cognitive performance. Due to the different types, frequencies, and lengths of AE employed in earlier studies, it was still unclear how AE affects MCI patient’s general cognition. Large-scale, rigorous, randomized control trials are required to investigate specific effect regions and mechanisms of action to identify the most suitable intervention strategies [28].
The current study found that the PD-CRS is a valid and reliable measure with a high level of specificity and sensitivity for diagnosing MCI and dementia in individuals with PD [21]. We also found that the MMSE cut-off rating 17 has 100% specificity and 81% sensitivity when evaluating cognitive impairment in adults [29].
Negative outcomes may be attributed to insufficient CBT dosage or duration, poor therapist-patient rapport or CBT adherence, and advanced dementia stage, limiting CBT effectiveness.
Conclusion
The study concluded that CBT is more effective than AE in improving attention and memory in individuals with dementia associated with PD. With its structured approach to addressing cognitive and emotional challenges, CBT offers superior benefits in enhancing cognitive functions compared to AE. These results underscore the importance of integrating therapeutic interventions that focus on cognitive and psychological aspects for managing cognitive impairments associated with dementia in PD. While AE has benefits, CBT should be considered a primary component of therapeutic strategies to improve this population’s cognitive function. Future research should explore the mechanisms underlying CBT’s effectiveness and investigate potential combined interventions that incorporate both CBT and AE for a more comprehensive approach to managing cognitive symptoms in dementia associated with PD. Cognitive behavioral therapy in PDD improves cognitive function, mood stabilization, and coping mechanisms. Potential negative consequences include emotional distress, therapist-patient mismatch, and insufficient progress.
The study’s strengths were that CBT techniques were non-invasive, simple and cost-effective, evidence-based approach, and easily administrated. The limitation of the study was that there was no objective assessment to evaluate cognitive impairment. Further research with larger sample sizes and longer intervention durations is warranted to confirm these findings because the smaller population may impact generalizability, including reduced statistical power to detect significant effects and limited representation of diverse population characteristics.
Ethical Considerations
Compliance with ethical guidelines
The study was done in accordance with the Ethics Committee on Human Experimentation of Institution ISRB No. 01/022/2023/ ISRB/PGSR/SCPT and with the 1975 Declaration of Helsinki, as revised in 2000.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Methodology, Writing - original draft: Dhanusia. S and Shareen Akbar. A; Investigation, writing, Review, and Editing: Dhanusia. S, Priyadharshani Kumar, and Vanitha Jayaraj; Supervision: Prathap Suganthirababu and Vignesh Srinivasan.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the referees for their useful suggestions and extend gratitude to the study participants who helped complete this research. The researcher thanks the authors of PD-CRS and MMSE.
References
Parkinson disease (PD) is the second most prevalent neurodegenerative disorder after Alzheimer disease. PD affected people of all races and ethnicities worldwide [1]. Globally, Parkinson disease affects approximately 1.51 individuals per 1000 people across all ages [2]; it is estimated that the prevalence of Parkinson disease dementia (PDD) is around 24%–31% [3].
Motor symptoms such as bradykinesia, stiffness, resting tremors, and irregularities in posture and gait were the first signs of PD [4]. Within the discipline of neuroscience, there was increased convergence in research on non-motor abnormalities like autonomic dysfunction, cognitive impairment, and psychiatric symptoms [5]. The features of cognitive impairment in PD can vary considerably in terms of which cognitive domains were affected, as well as in terms of the time of start and rate of development, much like the characteristics of motor symptoms [6]. The cognitive profile in PD had four problems of cognitive impairment: Attention and frontal executive function, memory, visuospatial skills, and language [7].
The cognitive impairment risk factor in PD was age factor (male>female), age of onset (>65), family history, psychiatric features, medication use (deprenyl, amantadine, agonist, anticholinergics, and cumulative exposure to levodopa), which resulted in increased mortality [8]. The investigation of cognitive decline in PD includes cerebrovascular fluid biomarkers, which showed aggregated α-synuclein, increased CSF tau protein, and reduced amount of β‐amyloid 1–42 and neuroimaging techniques such as magnetic resonance imaging and Positron emission tomography to detect brain pathology [9].
Dementia is a clinical syndrome characterized by acquired loss of emotional and cognitive abilities and increased mortality compared to non-dementia PD severe enough to interfere with daily functioning and quality of life [10]. Dementia affects 10% to 80% of individuals with PD at age 60, according to community-based studies, while the prevalence was only around 30% [11]. Up to 36% of PD sufferers with a recent diagnosis show cognitive impairment. The impact of dementia on the patient, the caregiver, and the wider community was significant [6]. Dementia and cognitive decline have an important clinical impact on PD [9]. Numerous research studies have conclusively shown their adverse impact on patient quality of life and the burden on care receivers and caregivers, and specialized therapeutic approaches are needed [12].
Attention is the process of filtering information related to external and internal stimuli modulated by the prefrontal cortex, which can be classified into simple and complex attention [13]. Memory was not a single, cohesive concept. Instead, various memory systems in different forms are supported by multiple neural systems of brain areas that could save data and access it later [14]. It is an essential physiological function that depends on medial temporal structures and is required for survival [13]. Memory is classified into emotional memory, implicit memory, and explicit memory. The amygdala, hippocampus, and other brain regions are necessary for emotional and explicit memory. The basal ganglia, motor cortex, and cerebellum support implicit memory [7].
Cognitive behavioral therapy (CBT) involves a set of clinical interventions designed to improve non-motor cognitive impairment, such as attention flexibility, memory, and behavior changes. CBT is an efficient, time-limited, cost-effective, and brief treatment. CBT has been designed to treat specific symptoms and behavioral patterns of the patient in as few as 10-20 sessions through assisted therapy programs, group format, self-help materials, and bibliography [15]. The program for CBT enhances the training of the brain’s prefrontal executive functions, working memory, and attention [16].
Aerobic exercise (AE) increases fitness, helps maintain good cognitive function, and benefits older people [17]. Additionally, physical activity has advantageous impacts on physiological functions, including glucose management and cardiovascular health, which increases the chance of Alzheimer disease and cognitive decline when impaired [18]. Aerobic exercise impacts cognitive functions such as working memory, inhibition, multitasking, planning, and selective attention, and these effects could be more prominent in older women than in older males. AE improves global cognitive functions such as memory and attention in mild cognitive impairment patients [19, 20].
CBT targets cognitive restructuring and emotional regulation, while AE enhances physical health and neuroplasticity. Comparing these interventions is crucial to determine whether one is superior or combining both yields synergistic benefits for mental health and cognitive functioning. This research will tip off clinicians’ evidence-based treatment decisions for optimal patient outcomes.
The research aimed to determine the effectiveness of CBT and AE on memory and attention in patients with dementia-related PD.
2. Materials and Methods
Study design and participants
A pilot study was conducted on PD patients with dementia in a private setting. The study was explained to all 30 participants, and their informed consent was obtained. A total of 30 participants were randomly assigned to groups A (n=15) and B (n=15) using a sealed envelope method. Eligible participants completed a pre-test evaluation, and values were recorded. For 6 weeks, group A received 30 minutes of cognitive behavioral therapy every day, while group B engaged in daily aerobic exercise for the same duration. A post-test outcome measure was recorded following the 6-week intervention.
Inclusion criteria
The inclusion criteria included both genders (male and female), above 60 years of age, at least 5 years of having PDD, mild to moderate cognitive impairment such as memory and attention with a mini-mental state examination (MMSE) score of less than 23, and Parkinson disease-cognitive rating scale (PD-CRS) less than 101, and agreement to cooperate with the treatment and follow up.
Exclusion criteria
The exclusion criteria included age below 60, diagnosis of dementia due to causes other than PD, MMSE score below 10, severe psychiatric disorders, participants with severe motor dysfunction, having cardiovascular disease, and not interested in participating in the study. Outcome measures were the PD-CRS and MMSE.
Assessment measures
Parkinson disease-cognitive rating scale (PD-CRS)
The PD-CRS is a reliable screening tool for identifying PD-related cognitive impairment. It consists of 2 cortical items, clock copying (scores 0–10) and confrontation naming (scores 0–20), and 7 frontal-subcortical items: Working memory (scores 0–10), sustained attention, clock drawing, alternating and action verbal fluencies (scores 0–20 and 0–30, respectively), and immediate and delayed free-recall verbal memory (scores 0–12 for both). All item scores are added up to produce a PD-CRS score (0–134), which is split into cortical (0–30) and frontal-subcortical (0–104) scores. PD-CRS has demonstrated exceptional clinical utility, high test-retest reliability (ICC>0.90), high inter-rater reliability (ICC>0.90), and strong internal consistency (α=0.94) [21].
Mini-mental state examination (MMSE)
The MMSE consists of 11 items that assess orientation, registration, attention or calculation (spelling or serial sevens), recall, naming, repetition, comprehension (written and verbal), writing, and construction. The total score ranges from 0 to 30, with a 25 or higher representing normal cognitive status. The scores are categorized as follows: Severe (0–17), mild (18–23), and normal (24–30).
The MMSE-I (modified MMSE) has demonstrated exceptional diagnostic accuracy, with a sensitivity of 99.0%-100.0%, specificity of 98.5%-97.0%, and area under the curve of 1.0/1.0. The internal consistency reliability of the MMSE-I was high (the Cronbach α=0.70) [22].
Study procedure
Eligible participants completed a pre-test evaluation, and values were recorded. Group A underwent 30 minutes a day of cognitive behavioral therapy for 6 weeks, and Group B underwent daily aerobic exercise for 6 weeks. A post-test treatment outcome measure was recorded following a 6-week intervention.
Memory and attention adaptation training
Memory and attention adaptation training (MAAT) was a brief CBT aimed at improving cognitive failure, such as memory and attention in daily living, and enhancing the overall quality of life of the patient. MAAT was given through offline mode in the private setting daily for 6 weeks with a session of 30 minutes. MAAT consists of four steps: Education, self-awareness and monitoring, self-regulation, and compensatory strategies.
The first step, education, begins by educating patients about common memory and attention challenges associated with PD and dementia. It highlights how negative thought patterns can impact daily life. It provides an interactive session where patients share specific examples of challenging tasks, such as misplacing items or forgetting appointments. Then, it introduces the concept of cognitive lapses and emphasizes how external distractions or internal stress can interfere with performance. The education step guides setting small, manageable daily goals, like remembering to take medications on time. It creates a weekly planner for tracking simple tasks and engages patients in cognitive activities such as crossword puzzles and word association exercises. The session is concluded by reflecting on the progress made in recognizing cognitive challenges.
The second step is self-awareness and monitoring. It involves practical exercises and guides patients in identifying internal and external distractions using mock scenarios, such as multitasking while organizing tasks. It teaches the importance of monitoring eating habits and documenting emotions or distractions that affect attention. In this step, templates are provided for tracking meal timings and emotional states, encouraging patients to observe their impact on focus. It assigns a take-home task where patients record two to three memory lapses or attention drifts. Finally, more challenging cognitive activities such as Sudoku or chess are introduced to enhance concentration and engagement.
The third step is self-regulation by teaching relaxation techniques, such as diaphragmatic breathing, to calm the mind encouraging patients to practice slow, deep breathing for 10 minutes daily while focusing on their breath. Then, progressive muscle relaxation is introduced. It targets specific muscle groups to reduce physical and emotional tension. The role of relaxation is discussed in alleviating stress and improving concentration. Patients will practice these techniques and reflect on their effects on emotional regulation and focus during the sessions.
The fourth step is compensatory strategies, comprising memory and attention management. Compensatory techniques are introduced to help patients manage memory and attention challenges better. Then, verbal rehearsal is taught, such as repeating steps aloud while completing tasks like preparing tea. The patients are trained using organizational tools like day planners or calendars to simplify daily routines. It works with patients to plan a mock weekly schedule, incorporating designated time slots for meals, exercise, and relaxation. Training is also provided on digital reminders, alarms, and visual cues to improve task management. Patients will practice setting up these aids on their devices during the session.
Finally, there are integration and goal achievement, consolidation, and reflection. The strategies learned over the past five weeks are reviewed and integrated in this step. Then, patients’ most effective techniques are discussed, and personalized adaptations are encouraged for future use. Group-based problem-solving activities such as collaborative puzzles or memory games are conducted to foster engagement and reinforce learned skills. Ultimately, individual and group achievements are celebrated, and patients are encouraged to maintain these practices in their daily routines.
PD-CRS and MMSE are suitable outcome measures for MAAT due to their specificity, comprehensiveness, and sensitivity to cognitive decline, standardization, brief administration, and vast establishment [23, 24].
Aerobic exercise
Aerobic exercise consists of a 6-week daily session for 30 minutes in a private setting. Aerobic exercise involves the first 5 min for warm-up and gentle stretching targeting specific muscle groups of the upper and lower extremities and 20 min of continuous cycling on a stationary bicycle with maximum speed, which refers to the highest achievable speed by an individual, typically measured in revolutions per minute (RPM) with the initial target of 50% maximum heart rate during the first week (60%-70% RPM) and then increased gradually to 75% by the sixth week (90% RPM). The maximum heart rate is calculated using the formula 220-Age, and the last 5 min is a cool-down period. If the participant has a target heart rate of <50%, alternative strategies like brisk walking and resistance exercises can be considered. The protocol’s effectiveness will be assessed through maximum oxygen uptake (VO2 max), muscle strength and endurance, and quality of life (evaluated using the 36-item short-form survey questionnaire).
Results
A sample of 30 was analyzed, data were collected from the participants using PD-CRS and MMSE, and pre-test and post-test values were noted after CBT and AE intervention. The result showed a P<0.001 and was considered more statistically significant with CBT than AE.
Group A pre-test and post-test values of MMSE (paired t-test)
CBT intervention significantly improved memory and attention in participants, as measured by MMSE (pre-test: 17.8±2.90, post-test: 27.2±2.64, P<0.001) (Table 1).

Group B pre-test and post-test values of MMSE (paired t-test)
AE intervention significantly improved memory and attention in participants, as measured by MMSE (pre-test: 17.8±2.90, post-test: 27.2±2.64, P<0.001) (Table 2).

Group A and Group B post-test of MMSE (independent t-test)
Both groups showed significant improvements from the pre-test to the post-test. However, group A (CBT) had significantly greater improvements compared to group B (AE) where P<0.001. The effect size calculated is 1.72, indicating a substantial difference between groups A and B (Table 3).

Group A pre-test and post-test values of PD-CRS (paired t-test)
CBT intervention significantly improved memory and attention in participants, as measured by PD-CRS (pre-test: 88.07±4.55, post-test: 92.07±4.95, P<0.001) (Table 4).

Group B pre-test and post-test values of PD-CRS (paired t-test)
AE intervention did not significantly improve memory and attention in participants, as measured by PD-CRS (pre-test: 86.47±3.83, post-test: 91.00±22.70, P=0.47) (Table 5).

Group A and Group B post-test values of PD-CRS (independent t-test)
Group A significantly improved from pre-test to post-test, while group B did not. There was no significant difference between the groups in post-test scores, indicating that both interventions had similar effects on PD-CRS outcomes where P=0.93. The calculated effect size is 0.98, indicating a large effect size and a strong difference between groups A and B (Table 6).

Discussion
The present study assessed the effectiveness of cognitive behavioral therapy and aerobic exercise in memory and attention in dementia-associated PD. Generally, PD is associated with dementia, and patients have impaired cognitive function. The present study also showed improved cognitive function, and CBT was more effective and beneficial than aerobic exercise.
AE’s cognitive benefits are less consistent and generalized than CBT, and PDD patients may struggle with AE due to motor symptoms, fatigue, and balance issues. Due to this reason, CBT is considered superior to AE [25, 26].
Our findings are supported by another study indicating that CBT effectively sustains attention among children with specific learning disorders. However, this intervention does not affect working and sustained memory in children with specific learning disorders.
Previous research reveals that those with long-term breast cancer who had undergone a brief program of CBT to manage the cognitive side effects of chemotherapy report better quality of life, improved daily cognitive function, and performance on neuropsychological tests. Participants reported high levels of treatment satisfaction and assessed MAAT as beneficial for learning and implementing cognitive compensatory methods to enhance abilities to make up for memory issues [27].
According to our research, individuals with mild cognitive impairment (MCI) who engaged in AE show improvement in their overall cognitive function and memory [19]. According to recent studies, AE was used as a practice that was both cost-effective and linked to a variety of physical advantages. According to the study’s findings, some people with MCI might also improve cognitively from exercise. For executive control tasks, AE has the strongest cognitive-improving effects in women. The at-risk group’s cognitive function could be enhanced with just 6 months of behavioral therapy using periodic increases in heart rate without the expense or side effects of most pharmaceutical treatments [20].
Recent studies show that AE could help older MCI patients with general cognitive performance. Due to the different types, frequencies, and lengths of AE employed in earlier studies, it was still unclear how AE affects MCI patient’s general cognition. Large-scale, rigorous, randomized control trials are required to investigate specific effect regions and mechanisms of action to identify the most suitable intervention strategies [28].
The current study found that the PD-CRS is a valid and reliable measure with a high level of specificity and sensitivity for diagnosing MCI and dementia in individuals with PD [21]. We also found that the MMSE cut-off rating 17 has 100% specificity and 81% sensitivity when evaluating cognitive impairment in adults [29].
Negative outcomes may be attributed to insufficient CBT dosage or duration, poor therapist-patient rapport or CBT adherence, and advanced dementia stage, limiting CBT effectiveness.
Conclusion
The study concluded that CBT is more effective than AE in improving attention and memory in individuals with dementia associated with PD. With its structured approach to addressing cognitive and emotional challenges, CBT offers superior benefits in enhancing cognitive functions compared to AE. These results underscore the importance of integrating therapeutic interventions that focus on cognitive and psychological aspects for managing cognitive impairments associated with dementia in PD. While AE has benefits, CBT should be considered a primary component of therapeutic strategies to improve this population’s cognitive function. Future research should explore the mechanisms underlying CBT’s effectiveness and investigate potential combined interventions that incorporate both CBT and AE for a more comprehensive approach to managing cognitive symptoms in dementia associated with PD. Cognitive behavioral therapy in PDD improves cognitive function, mood stabilization, and coping mechanisms. Potential negative consequences include emotional distress, therapist-patient mismatch, and insufficient progress.
The study’s strengths were that CBT techniques were non-invasive, simple and cost-effective, evidence-based approach, and easily administrated. The limitation of the study was that there was no objective assessment to evaluate cognitive impairment. Further research with larger sample sizes and longer intervention durations is warranted to confirm these findings because the smaller population may impact generalizability, including reduced statistical power to detect significant effects and limited representation of diverse population characteristics.
Ethical Considerations
Compliance with ethical guidelines
The study was done in accordance with the Ethics Committee on Human Experimentation of Institution ISRB No. 01/022/2023/ ISRB/PGSR/SCPT and with the 1975 Declaration of Helsinki, as revised in 2000.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Methodology, Writing - original draft: Dhanusia. S and Shareen Akbar. A; Investigation, writing, Review, and Editing: Dhanusia. S, Priyadharshani Kumar, and Vanitha Jayaraj; Supervision: Prathap Suganthirababu and Vignesh Srinivasan.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the referees for their useful suggestions and extend gratitude to the study participants who helped complete this research. The researcher thanks the authors of PD-CRS and MMSE.
References
- Fang C, Lv L, Mao S, Dong H, Liu B. Cognition Deficits in Parkinson's Disease: Mechanisms and Treatment. Parkinson's Disease. 2020; 2020:2076942. [DOI:10.1155/2020/2076942] [PMID] [PMCID]
- Severiano E, Sousa C, Alarcão J, Pavão Martins I, Ferreira JJ. Frequency of dementia in Parkinson's disease: A systematic review and meta-analysis. Journal of The Neurological Sciences. 2022; 432:120077. [DOI:10.1016/j.jns.2021.120077] [PMID]
- Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nature Reviews. Disease Primers 2021; 7(1):47. [DOI:10.1038/s41572-021-00280-3] [PMID]
- Balestrino R, Schapira AHV. Parkinson disease. European Journal of Neurology. 2020; 27(1):27-42. [DOI:10.1111/ene.14108] [PMID]
- Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. Journal of Neurochemistry. 2016; 139(Suppl 1):318-24. [DOI:10.1111/jnc.13691] [PMID]
- Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nature Reviews Disease Primers. 2021; 7(1):47. [DOI:10.1038/s41572-021-00280-3] [PMID]
- Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson’s disease. Brain. Pathology. 2010; 20(3):640-5. [DOI:10.1111/j.1750-3639.2010.00373.x] [PMID]
- Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009; 73(18):1469-77. [DOI:10.1212/WNL.0b013e3181bf992f] [PMID]
- Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Natural Reviews Neurology. 2017; 13(4):217-31. [DOI:10.1038/nrneurol.2017.27] [PMID]
- Geldmacher DS, Whitehouse PJ. Evaluation of dementia. The New England Journal of Medicine. 1996; 335(5):330-6. [DOI:10.1056/NEJM199608013350507] [PMID]
- Zhu J, Cui Y, Zhang J, Yan R, Su D, Zhao D, et al. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: A systematic review and meta-analysis. The Lancet Healthy Longevity. 2024; 5(7):e464-79. [DOI:10.1016/S2666-7568(24)00094-1] [PMID]
- Rosqvist K, Schrag A, Odin P, The CLaSP Consortium. Caregiver Burden and Quality of Life in Late Stage Parkinson's Disease. Brain Sciences. 2022; 12(1):111. [DOI:10.3390/brainsci12010111] [PMID]
- Bisaz R, Travaglia A, Alberini CM. The neurobiological bases of memory formation: From physiological conditions to psychopathology. Psychopathology. 2014; 47(6):347-56. [DOI:10.1159/000363702] [PMID] [PMCID]
- Young JZ. The memory system of the brain. California: University of California Press; 2023. [DOI:10.2307/jj.8306259]
- Bond FW, Dryden W. Handbook of brief cognitive behaviour therapy. New Jersey: John Wiley & Sons; 2005. [Link]
- Virta M, Salakari A, Antila M, Chydenius E, Partinen M, Kaski M. et al. Short cognitive behavioral therapy and cognitive training for adults with ADHD-a randomized controlled pilot study. Neuropsychiatric Disease and Treatement. 2010; 6:443-53. [DOI:10.2147/ndt.s11743] [PMID] [PMCID]
- Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2015; 2015(4):CD005381. [PMID]
- Sabia S, Dugravot A, Dartigues JF, Abell J, Elbaz A, Kivimäki M, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017; 357:j2709. [DOI:10.1136/bmj.j2709] [PMID]
- Zheng G, Xia R, Zhou W, Tao J, Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine. 2016; 50(23):1443-50. [DOI:10.1136/bjsports-2015-095699] [PMID]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Archives of Neurology. 2010; 67(1):71-9. [Link]
- Mahmoudi Asl A, Mehdizadeh M, Kulisevsky J, Sabet A, Taghavi Azar Sharabiani P, Mehdizadeh H, et al. Reliability, validity, and diagnostic accuracy of Parkinson’s Disease-Cognitive Rating Scale in Iranian patients with idiopathic Parkinson’s disease. Disability and Rehabilitation. 2022; 44(10):2091-8. [DOI:10.1080/09638288.2020.1813337] [PMID]
- Babacan-Yıldız G, Ur-Özçelik E, Kolukısa M, Işık AT, Gürsoy E, Kocaman G, et al. [Validity and Reliability Studies of Modified Mini Mental State Examination (MMSE-E) For Turkish Illiterate Patients With Diagnosis of Alzheimer Disease (Turkish)]. Turk Psikiyatri Dergisi = Turkish Journal of Psychiatry. 2016; 27(1):41-6. [PMID]
- McDonald BC, Flashman LA, Arciniegas DB, Ferguson RJ, Xing L, Harezlak J, et al. Methylphenidate and memory and attention adaptation training for persistent cognitive symptoms after traumatic brain injury: A randomized, placebo-controlled trial. Neuropsychopharmacology. 2017; 42(9):1766-75. [DOI:10.1038/npp.2016.261] [PMID]
- Ferguson R, Gillock K. Memory and Attention Adaptation Training: A brief cognitive behavioral therapy for cancer survivors: Clincian Manual. Oxford: Oxford University Press; 2021. [DOI:10.1093/med/9780197521571.001.0001]
- Zhen K, Zhang S, Tao X, Li G, Lv Y, Yu L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson's disease. NPJ Parkinson's Disease. 2022; 8(1):146. [DOI:10.1038/s41531-022-00418-4] [PMID]
- Wu X, Shi M, Lian Y, Zhang H. Cognitive behavioral therapy approaches to the improvement of mental health in Parkinson’s disease patients: A systematic review and meta-analysis. BMC Neurology. 2024; 24(1):352. [DOI:10.1186/s12883-024-03859-x] [PMID]
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007; 16(8):772-7. [DOI:10.1002/pon.1133] [PMID]
- Yong L, Liu L, Ding T, Yang G, Su H, Wang J, et al. Evidence of effect of aerobic exercise on cognitive intervention in older adults with mild cognitive impairment. Frontiers in Psychiatry. 2021; 12:713671. [DOI:10.3389/fpsyt.2021.713671] [PMID]
- Jain M, Passi GR. Assessment of a modified Mini-Mental Scale for cognitive functions in children. Indian Pediatrics. 2005; 42(9):907-12. [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/11/6 | Accepted: 2024/12/22 | Published: 2025/04/1
Received: 2024/11/6 | Accepted: 2024/12/22 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



