Fri, Jan 30, 2026
Volume 15, Issue 4 (Autumn 2025)
PTJ 2025, 15(4): 323-332 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fattahzadeh G, Amani F, Jangi F, Parkalian R. Demographic and Clinical Characteristics of Epilepsy Patients in Ardabil City, Iran, 2022. PTJ 2025; 15 (4) :323-332
URL: http://ptj.uswr.ac.ir/article-1-680-en.html
URL: http://ptj.uswr.ac.ir/article-1-680-en.html
1- Department of Neurology, Faculty of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
2- Department of Social Medicine and Statistics, Faculty of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
2- Department of Social Medicine and Statistics, Faculty of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
Keywords: Epilepsy, Seizures, Risk factors, Neurological disorders, Magnetic resonance imaging (MRI)
Full-Text [PDF 652 kb]
(245 Downloads)
| Abstract (HTML) (1267 Views)
Full-Text: (349 Views)
Introduction
Between 50 and 70 million people worldwide suffer from epilepsy, accounting for 0.75% of all diseases. With an estimated 2.4 million new cases annually, the incidence and prevalence are 50 per 100 000 and 700 per 100 000, respectively. In 2012, nearly 20.6 million disability-adjusted life years were lost due to epilepsy [1, 2]. Almost 80% of epilepsy sufferers reside in low- and middle-income countries, where the incidence rate can be up to double that of high-income nations. Three-quarters of patients with epilepsy in low-income areas do not receive requisite antiepileptic medications (AEDs), indicating a serious treatment gap. Nevertheless, with appropriate AED treatment, 60-70% of patients with epilepsy can lead normal lives [3-5].
Globally, the prevalence of epilepsy varies; in sub-Saharan Africa and Latin America, the highest lifetime prevalence is observed, but in Asia, it is comparable to that of Western nations. Prevalence rates are often higher in rural than in urban regions [6, 7]. Factors, such as the underlying causes of epilepsy, study populations, genetic and environmental factors, and variations in diagnostic capabilities and cultural perspectives, all affect the prevalence discrepancies between high- and low-income nations. With 23 million individuals with epilepsy, Asia has notable economic differences [8–10]. Infections of the central nervous system and other illnesses are widespread in Asia, contributing to higher incidence [11].
In contrast to the peaks in infancy and later age observed in industrialized nations, epilepsy in Asia has two peak onset ages: Childhood and early adulthood. Nonetheless, the childhood and old age peaks in Taiwan, Japan, and Thailand are comparable to those in affluent nations [12]. The peak age at which epilepsy onset occurs may be influenced by the fact that the overall population in Asia is younger than in industrialized nations. The International League Against Epilepsy classified the types of epilepsy and seizures that are reported in Asia as follows: Idiopathic epilepsy (4–42%), focal seizures (31–50%), symptomatic epilepsy (22–53%), generalized seizures (50–69%), and cryptogenic epilepsy (13–60%) [13–17].
Individuals with epilepsy are more likely to experience mental illnesses, which can lower their quality of life. Patients with epilepsy have been found to have higher rates of depression, anxiety, and suicidal ideation than the general population [18]. Psychiatric problems are frequently misdiagnosed and treated insufficiently, most likely as a result of clinician consultations that prioritize seizure management. The international league against epilepsy has created guidelines for treating anxiety and depression in people with epilepsy, and their acceptance in Asian healthcare systems is crucial [19].
Patients with epilepsy are also more likely to die young, especially in the first two years after diagnosis. Persistent seizures and symptomatic epilepsy are reliable indicators of early death [20]. Additionally, compared to the general community, patients with epilepsy in Bangladesh and Laos had significantly higher rates of injury-related mortality, including drowning. Reducing the risk of premature death in individuals with epilepsy requires improved seizure control, treatment of psychiatric comorbidities, and provision of seizure management education [21]. This study aimed to determine the clinical and demographic traits of epileptic patients who were sent to Alavi Hospital in Ardabil city in 2022.
Materials and Methods
The Neurology Department of Ardabil University of Medical Sciences conducted this descriptive cross-sectional study fromMarch 20, 2020, to March 20, 2022. The goal of this study was to analyze the clinical characteristics of patients with epilepsy who visited Alavi Hospital in Ardabil city during this period. The inclusion criteria included comprehensive medical records, a verified diagnosis of epilepsy, and frequent follow-up. Patients on incomplete treatment protocols, those receiving CNS drugs, those receiving therapy at different facilities, and those who did not finish treatment were excluded. Patients’ clinical and demographic information was gathered using a checklist.
Patients or their caregivers were interviewed to collect data, which included clinical information (disease duration, family history, comorbidities, risk factors, clinical symptoms, symptom severity and frequency, medication types, and dosages) and demographic information (age, sex, residence, occupation, education level, marital status, and economic status). SPSS software, version 25, was used for data entry and statistical analysis, and tables and charts were used to display the results.
Results
Using a census sample technique, the study included all eligible patients between 2020 and 2022. We examined the medical records of 400 individuals with epilepsy who visited the hospital’s neurology and emergency departments. After examining these 400 patients, the majority of participants (208) were found to be male. Participants ranged in age from 15 to 90 years, with an average age of 40.36±9.77 years. The majority of participants were economically disadvantaged, married, worked as freelancers, lived in cities, and had only completed high school. Table 1 presents the participants’ demographic and social information. Two age groups had the highest prevalence, according to an analysis of the age distribution at the onset of epilepsy: Those over 60 years (104 patients) and those between 20 and 25 years (72 patients). In other words, epilepsy had the highest frequency within these age groups.
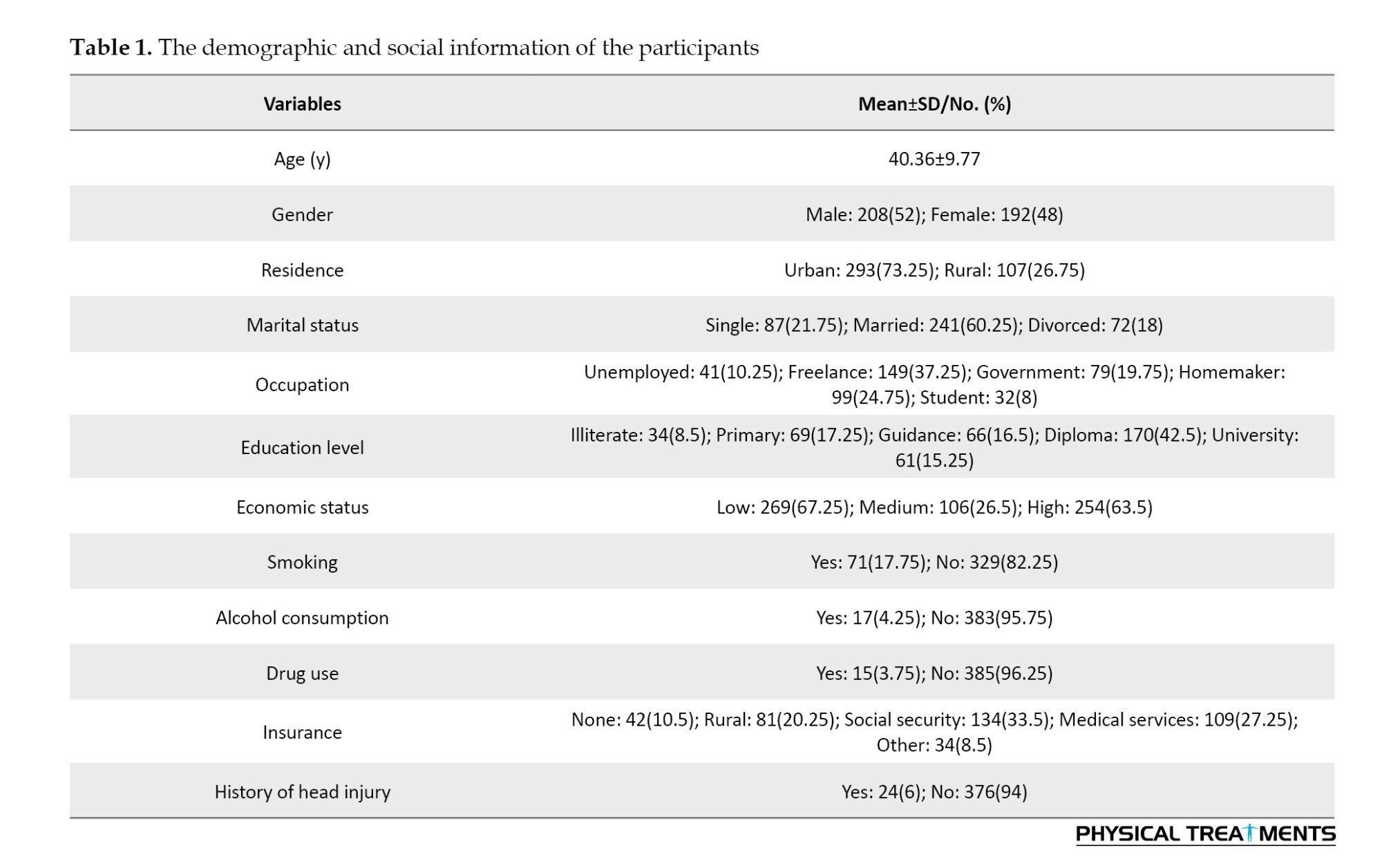
Diabetes (89 patients), mental illness (98 patients), and hypertension (104 patients) were the most common comorbidities among the study participants. Figure 1 shows the distribution of underlying conditions among the study participants.
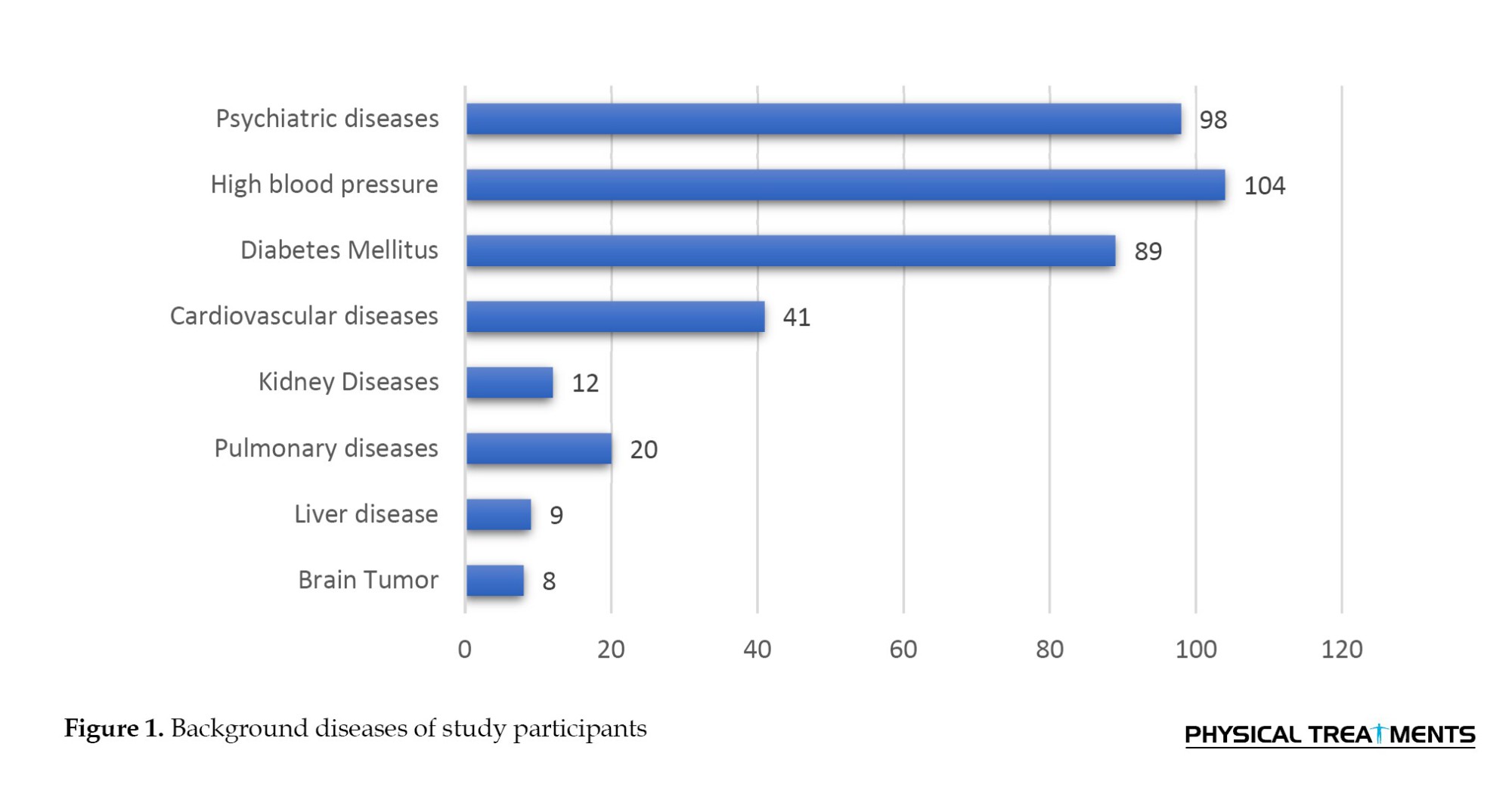
According to the study, 190 participants (the majority of the group) took only one medication. The participants’ risk factors for epilepsy were carefully recorded. The most common risk factors were a history of head injury (24 individuals) and a family history of epilepsy (27 individuals). The most prevalent clinical symptoms that participants reported were uncontrollable arm and leg movements in 181 people (45.25%), drooling during seizures in 226 people (56.5%), and loss of consciousness or awareness in 239 people (59.75%).Table 2 presents the details of the prevalence of these clinical symptoms.
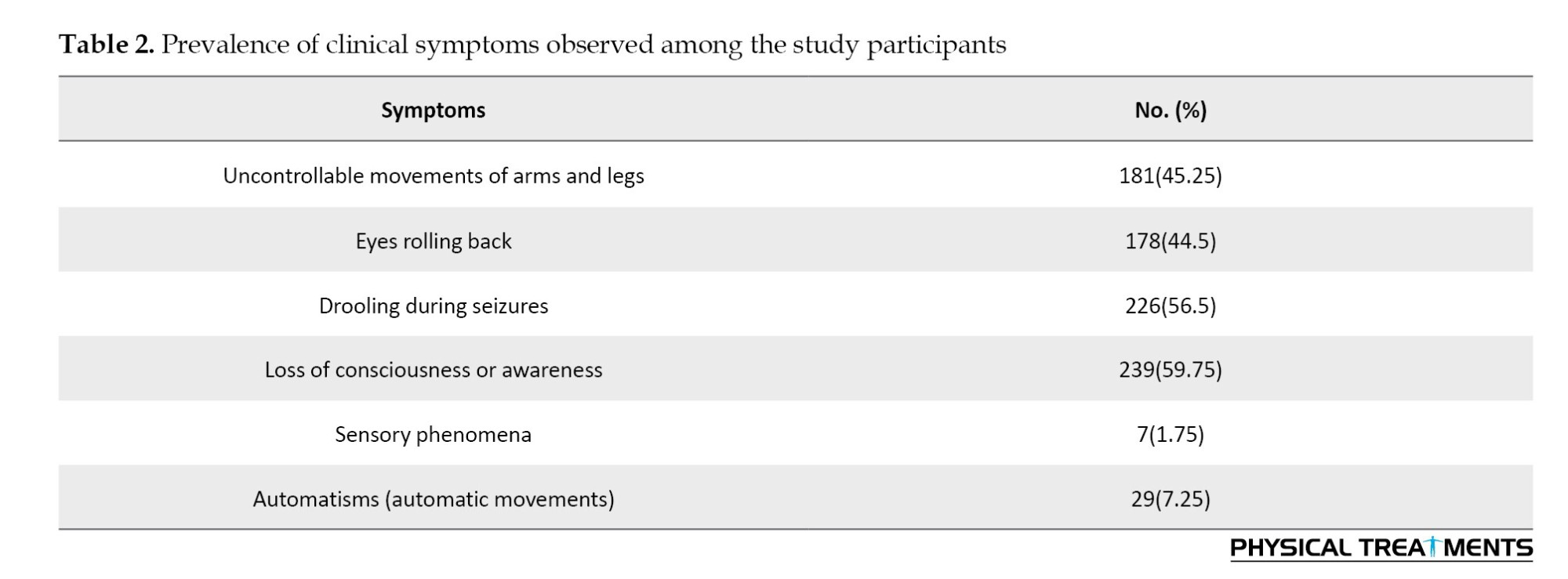
According to the study, the most frequent seizure types among patients were tonic-clonic seizures (160 cases) and focal seizures without loss of consciousness (37 cases). Figure 2 shows these findings. According to an analysis of participants’ seizure attack histories, 276 people (69%) had a history of epileptic seizures.
Participants’ MRI results revealed that 309 of them had no discernible abnormalities, with the most prevalent findings being gliosis and encephalomalacia in 27 participants. The participants’ EEG results showed that the majority of the findings were non-epileptiform (Table 3).
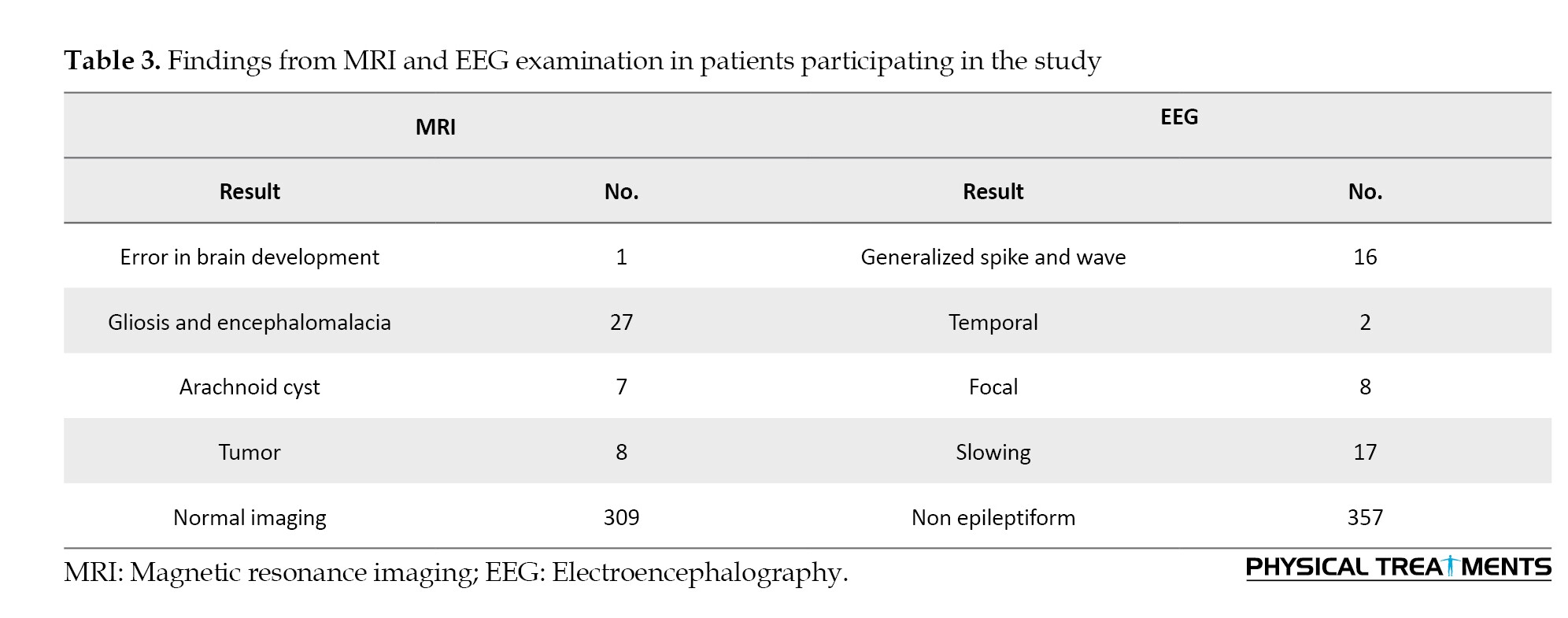
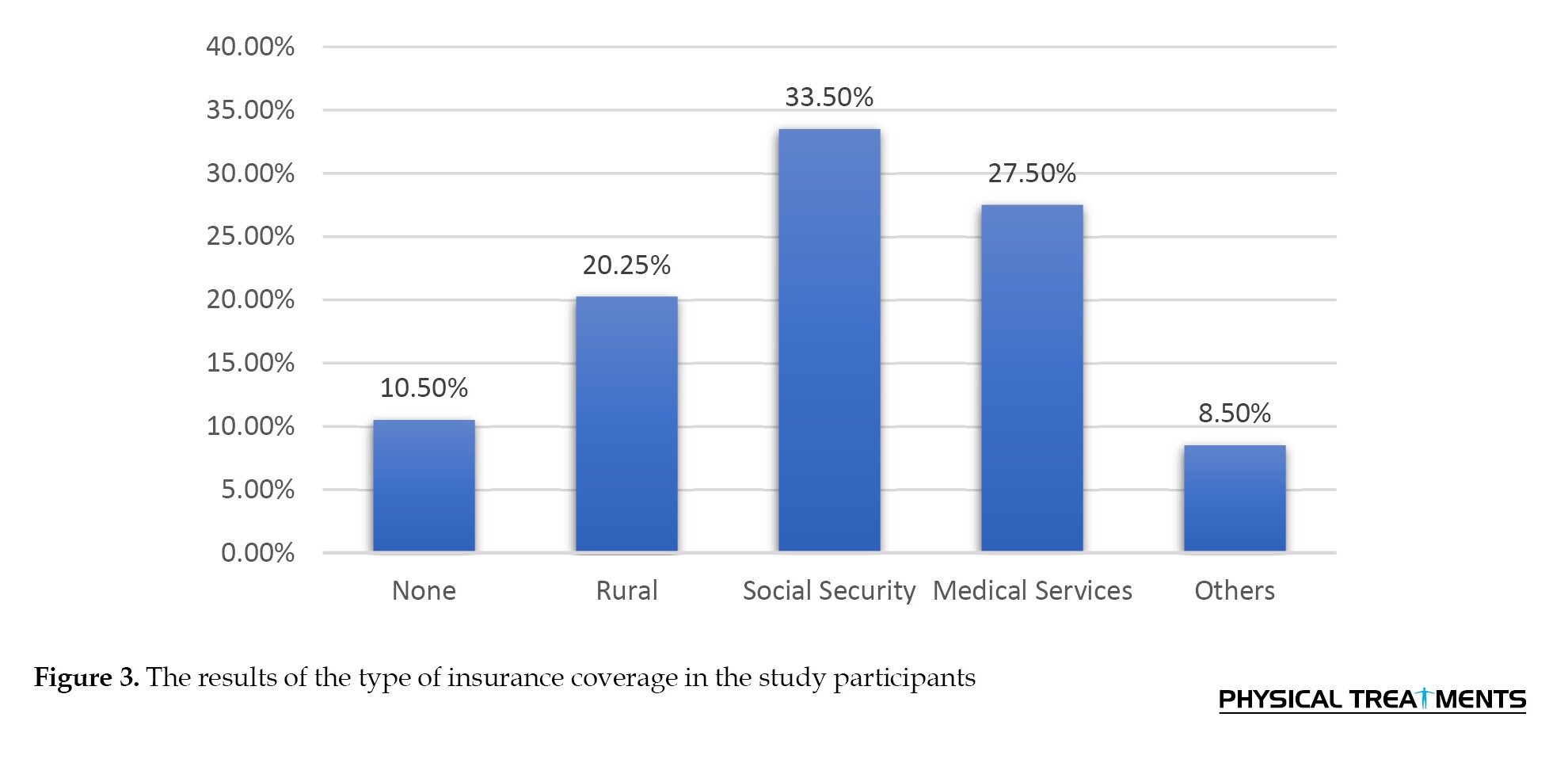
Only a small proportion of the individuals in the study reported using drugs, alcohol, or smoking. Regarding health insurance coverage, 134 participants (33.5%) were covered by social security insurance and 109 participants (27.25%) by medical services insurance. A history of head injury was reported by 24 participants (6%) (Figure 3).
Discussion
This study was conducted to investigate the clinical and demographic characteristics of patients with epilepsy who visited Alavi Hospital in Ardabil city in 2021. According to our findings, the two age groups with the highest prevalence were those over 60 (104 patients) and those between 20 and 25 (72 patients). Diabetes (89 patients), mental illness (98 patients), and hypertension (104 patients) were the most prevalent comorbidities. Most participants (190 patients) followed a single-drug regimen. The two most prevalent risk factors were a history of head trauma (24 patients) and a family history of epilepsy (27 patients). The most common forms were focal epilepsy without loss of consciousness (37 individuals) and tonic-clonic epilepsy (160 patients). Of these, 276 patients (69%) had a history of epileptic seizures. MRI results showed that the most common findings were Gliosis and Encephalomalacia in 27 patients. According to the EEG findings, the most common result was nonepileptiform.
To the best of our knowledge, this is the first study to examine the clinical and demographic characteristics of patients with epilepsy in the neurology department of Alavi Hospital, affiliated with Ardabil University of Medical Sciences. Two main challenges in prevalence studies are ensuring diagnostic accuracy and the completeness of available information. We made every effort to guarantee that few cases went undiagnosed and that we thoroughly and accurately investigated patients who had been diagnosed with epilepsy. Approximately 7% of people with overt epilepsy went undiagnosed, according to a US study that used a screening questionnaire in the general population. This percentage is probably lower here due to the state of medical care in Iran today and the growing awareness of epilepsy. Additionally, such cases are insufficient for planning healthcare resources.
The highest prevalence of epilepsy in our study was found in patients aged >60 years. Other prevalence studies of epilepsy in Iran and other regions have shown similar age distribution. Epidemiological studies frequently reveal that the prevalence of epilepsy rises with age; however, the extent of this increase is unknown. Lowenstein and Alldredge observed a prevalence of 14.8 per 1 000 in adults over 75 years old in Rochester, USA, in 1980 [15]. According to prospective research conducted by Alldredge et al. in the Netherlands, the frequency among those aged 65-85 years was 9.9-12.1 per 1 000 [16]. According to a study in Netherlands, the prevalence of induced seizures in people aged 70-74 is approximately 3 per 1000. The classification of seizures after a cerebrovascular event, a significant risk factor for seizures in the elderly, is primarily responsible for the greater prevalence in the elderly that our study found. These seizures are typically treated right away after an ischemic episode.
As previously noted by other researchers, the most prevalent type of seizure is tonic-clonic [21]. According to other studies, the prevalence of tonic-clonic epilepsy is 40%, which is in line with findings from Finland and Sweden (38% and 41%, respectively) [22, 23]. Due to clinical errors and the lack of paraclinical data, such as brain imaging results, half of the epilepsies that were first diagnosed as generalized are now categorized as secondary generalized epilepsy [20]. The higher frequency of epilepsy in males with an overall sex ratio of 1.5 may also be explained by the increased prevalence of symptomatic epilepsy in men, which is mostly attributable to head injury.
As previously stated, both the general population and those over 60 (the elderly) have higher rates of epileptic seizures. Furthermore, research from several nations indicates that older adults are more likely to experience their first epileptic seizure [24–26]. According to earlier research, seizures occur differently in young and old individuals. Younger people are more likely to experience generalized seizures, whereas older people are more likely to experience focal seizures [27]. According to our study, generalized seizures are more common in younger persons than in adults over 60, which is consistent with other studies [11, 28, 29]. However, individuals aged > 60 years are more likely to experience subsequent generalized and mixed seizures [22]. According to earlier research, the majority of seizures in young people originate in the temporal lobes; however, seizures that originate outside of the temporal lobes are more common in older individuals [8, 11, 23]. The different anatomical causes of seizures in these two age groups account for the observed discrepancy.
MRI findings in the study participants showed that the most common finding was Gliosis and Encephalomalacia in 27 patients. This suggests a strong correlation between these anomalies and epilepsy symptoms in the population under study. Identifying these recurring trends could impact the diagnosis of epilepsy and help comprehend its underlying neurobiological causes.
We compared our results with those of previous studies to provide context. The relationship between epilepsy and MRI results has been the subject of numerous studies, and our findings are consistent with some of the prevalent patterns. For example, gliosis and encephalomalacia anomalies were highly prevalent in the groups of patients with epilepsy studied by Sillanpää et al. [30] and Brodie et al. [26]. It is crucial to remember that variations in sample size, demographics, and imaging procedures can cause disparities in research findings. Different abnormalities may be highlighted in different research, or differing prevalence rates may be reported.
We contribute to the ongoing discussion in this area and provide a more comprehensive understanding of the various MRI findings associated with epilepsy by integrating our findings with the existing literature [19, 26, 31]. This comparative analysis contributes to the body of knowledge in this field and opens the door to better methods of diagnosis and treatment for patients with epilepsy.
According to our research, the most prevalent subtype of epilepsy was tonic-clonic epilepsy, which involved 160 individuals, whereas 37 patients had focal epilepsy without unconsciousness. These results clarify the distribution of epilepsy subtypes in the study sample. We compared our findings with those of previous studies on the prevalence of epilepsy subgroups to corroborate our results. Our research supports several earlier studies that consistently identified tonic-clonic epilepsy as one of the most prevalent subtypes [23, 25, 32]. Due to its unique and identifiable characteristics, which frequently result in a timely diagnosis, tonic-clonic epilepsy is very common. Our study’s prevalence of focal epilepsy without loss of consciousness, however, seems to be slightly lower than that reported in earlier studies. Differences in study populations, geographic regions, and diagnostic criteria may have caused this disparity. The symptoms of focal epilepsy without loss of consciousness are frequently mild and transient, making precise diagnosis difficult and frequently missed by many friends.
Conclusion
According to this study, tonic-clonic and focal epilepsy were the most prevalent forms of epilepsy in Ardabil County among adults. Interestingly, fewer focal instances were found than anticipated. Thus, it is essential to do more accurate MRIs, repeat and extend EEGs, and inform caregivers and medical professionals about focal seizure symptoms, particularly in elderly individuals. This method may result in a more precise diagnosis of seizure types and, as a result, more successful treatments. In addition to improving healthcare services for surgery or other treatments in drug-resistant epilepsy, these data can help estimate the target population for novel AEDs.
Limitations of the study
Our study had several limitations, which are outlined as follows: It was a single-center retrospective study. There was no precise information on the regular use of prescribed medications by patients. This study did not include long-term follow-up (beyond one year). Additionally, in most cases, the type of seizure was not observed by the treating physicians.
Strengths of the study
This study’s strengths include its large, representative sample and comprehensive data collection, covering key demographic and clinical factors, such as comorbidities and risk factors. The use of standardized questionnaires and consistent clinical evaluations ensured the reliability of the results. Additionally, focusing on MRI and EEG findings provides deeper insights into the neurological aspects of epilepsy. These strengths make the study valuable for healthcare providers and policymakers, aiding in better understanding and management of epilepsy in the Ardabil region
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research Ethics Committee of Ardabil University of Medical Sciences (Code: IR.ARUMS.REC.1401.134).
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
We extend our sincere gratitude to the staff and administration of Alavi Hospital in Ardabil city, Iran, for their assistance and support during the data collection phase of this study. We are also grateful to the patients and their families for their participation and cooperation. Additionally, we acknowledge the contributions of Ardabil University of Medical Sciences for providing ethical approval and necessary resources to conduct this research.
References
Between 50 and 70 million people worldwide suffer from epilepsy, accounting for 0.75% of all diseases. With an estimated 2.4 million new cases annually, the incidence and prevalence are 50 per 100 000 and 700 per 100 000, respectively. In 2012, nearly 20.6 million disability-adjusted life years were lost due to epilepsy [1, 2]. Almost 80% of epilepsy sufferers reside in low- and middle-income countries, where the incidence rate can be up to double that of high-income nations. Three-quarters of patients with epilepsy in low-income areas do not receive requisite antiepileptic medications (AEDs), indicating a serious treatment gap. Nevertheless, with appropriate AED treatment, 60-70% of patients with epilepsy can lead normal lives [3-5].
Globally, the prevalence of epilepsy varies; in sub-Saharan Africa and Latin America, the highest lifetime prevalence is observed, but in Asia, it is comparable to that of Western nations. Prevalence rates are often higher in rural than in urban regions [6, 7]. Factors, such as the underlying causes of epilepsy, study populations, genetic and environmental factors, and variations in diagnostic capabilities and cultural perspectives, all affect the prevalence discrepancies between high- and low-income nations. With 23 million individuals with epilepsy, Asia has notable economic differences [8–10]. Infections of the central nervous system and other illnesses are widespread in Asia, contributing to higher incidence [11].
In contrast to the peaks in infancy and later age observed in industrialized nations, epilepsy in Asia has two peak onset ages: Childhood and early adulthood. Nonetheless, the childhood and old age peaks in Taiwan, Japan, and Thailand are comparable to those in affluent nations [12]. The peak age at which epilepsy onset occurs may be influenced by the fact that the overall population in Asia is younger than in industrialized nations. The International League Against Epilepsy classified the types of epilepsy and seizures that are reported in Asia as follows: Idiopathic epilepsy (4–42%), focal seizures (31–50%), symptomatic epilepsy (22–53%), generalized seizures (50–69%), and cryptogenic epilepsy (13–60%) [13–17].
Individuals with epilepsy are more likely to experience mental illnesses, which can lower their quality of life. Patients with epilepsy have been found to have higher rates of depression, anxiety, and suicidal ideation than the general population [18]. Psychiatric problems are frequently misdiagnosed and treated insufficiently, most likely as a result of clinician consultations that prioritize seizure management. The international league against epilepsy has created guidelines for treating anxiety and depression in people with epilepsy, and their acceptance in Asian healthcare systems is crucial [19].
Patients with epilepsy are also more likely to die young, especially in the first two years after diagnosis. Persistent seizures and symptomatic epilepsy are reliable indicators of early death [20]. Additionally, compared to the general community, patients with epilepsy in Bangladesh and Laos had significantly higher rates of injury-related mortality, including drowning. Reducing the risk of premature death in individuals with epilepsy requires improved seizure control, treatment of psychiatric comorbidities, and provision of seizure management education [21]. This study aimed to determine the clinical and demographic traits of epileptic patients who were sent to Alavi Hospital in Ardabil city in 2022.
Materials and Methods
The Neurology Department of Ardabil University of Medical Sciences conducted this descriptive cross-sectional study fromMarch 20, 2020, to March 20, 2022. The goal of this study was to analyze the clinical characteristics of patients with epilepsy who visited Alavi Hospital in Ardabil city during this period. The inclusion criteria included comprehensive medical records, a verified diagnosis of epilepsy, and frequent follow-up. Patients on incomplete treatment protocols, those receiving CNS drugs, those receiving therapy at different facilities, and those who did not finish treatment were excluded. Patients’ clinical and demographic information was gathered using a checklist.
Patients or their caregivers were interviewed to collect data, which included clinical information (disease duration, family history, comorbidities, risk factors, clinical symptoms, symptom severity and frequency, medication types, and dosages) and demographic information (age, sex, residence, occupation, education level, marital status, and economic status). SPSS software, version 25, was used for data entry and statistical analysis, and tables and charts were used to display the results.
Results
Using a census sample technique, the study included all eligible patients between 2020 and 2022. We examined the medical records of 400 individuals with epilepsy who visited the hospital’s neurology and emergency departments. After examining these 400 patients, the majority of participants (208) were found to be male. Participants ranged in age from 15 to 90 years, with an average age of 40.36±9.77 years. The majority of participants were economically disadvantaged, married, worked as freelancers, lived in cities, and had only completed high school. Table 1 presents the participants’ demographic and social information. Two age groups had the highest prevalence, according to an analysis of the age distribution at the onset of epilepsy: Those over 60 years (104 patients) and those between 20 and 25 years (72 patients). In other words, epilepsy had the highest frequency within these age groups.

Diabetes (89 patients), mental illness (98 patients), and hypertension (104 patients) were the most common comorbidities among the study participants. Figure 1 shows the distribution of underlying conditions among the study participants.

According to the study, 190 participants (the majority of the group) took only one medication. The participants’ risk factors for epilepsy were carefully recorded. The most common risk factors were a history of head injury (24 individuals) and a family history of epilepsy (27 individuals). The most prevalent clinical symptoms that participants reported were uncontrollable arm and leg movements in 181 people (45.25%), drooling during seizures in 226 people (56.5%), and loss of consciousness or awareness in 239 people (59.75%).Table 2 presents the details of the prevalence of these clinical symptoms.

According to the study, the most frequent seizure types among patients were tonic-clonic seizures (160 cases) and focal seizures without loss of consciousness (37 cases). Figure 2 shows these findings. According to an analysis of participants’ seizure attack histories, 276 people (69%) had a history of epileptic seizures.

Participants’ MRI results revealed that 309 of them had no discernible abnormalities, with the most prevalent findings being gliosis and encephalomalacia in 27 participants. The participants’ EEG results showed that the majority of the findings were non-epileptiform (Table 3).

Only a small proportion of the individuals in the study reported using drugs, alcohol, or smoking. Regarding health insurance coverage, 134 participants (33.5%) were covered by social security insurance and 109 participants (27.25%) by medical services insurance. A history of head injury was reported by 24 participants (6%) (Figure 3).

Discussion
This study was conducted to investigate the clinical and demographic characteristics of patients with epilepsy who visited Alavi Hospital in Ardabil city in 2021. According to our findings, the two age groups with the highest prevalence were those over 60 (104 patients) and those between 20 and 25 (72 patients). Diabetes (89 patients), mental illness (98 patients), and hypertension (104 patients) were the most prevalent comorbidities. Most participants (190 patients) followed a single-drug regimen. The two most prevalent risk factors were a history of head trauma (24 patients) and a family history of epilepsy (27 patients). The most common forms were focal epilepsy without loss of consciousness (37 individuals) and tonic-clonic epilepsy (160 patients). Of these, 276 patients (69%) had a history of epileptic seizures. MRI results showed that the most common findings were Gliosis and Encephalomalacia in 27 patients. According to the EEG findings, the most common result was nonepileptiform.
To the best of our knowledge, this is the first study to examine the clinical and demographic characteristics of patients with epilepsy in the neurology department of Alavi Hospital, affiliated with Ardabil University of Medical Sciences. Two main challenges in prevalence studies are ensuring diagnostic accuracy and the completeness of available information. We made every effort to guarantee that few cases went undiagnosed and that we thoroughly and accurately investigated patients who had been diagnosed with epilepsy. Approximately 7% of people with overt epilepsy went undiagnosed, according to a US study that used a screening questionnaire in the general population. This percentage is probably lower here due to the state of medical care in Iran today and the growing awareness of epilepsy. Additionally, such cases are insufficient for planning healthcare resources.
The highest prevalence of epilepsy in our study was found in patients aged >60 years. Other prevalence studies of epilepsy in Iran and other regions have shown similar age distribution. Epidemiological studies frequently reveal that the prevalence of epilepsy rises with age; however, the extent of this increase is unknown. Lowenstein and Alldredge observed a prevalence of 14.8 per 1 000 in adults over 75 years old in Rochester, USA, in 1980 [15]. According to prospective research conducted by Alldredge et al. in the Netherlands, the frequency among those aged 65-85 years was 9.9-12.1 per 1 000 [16]. According to a study in Netherlands, the prevalence of induced seizures in people aged 70-74 is approximately 3 per 1000. The classification of seizures after a cerebrovascular event, a significant risk factor for seizures in the elderly, is primarily responsible for the greater prevalence in the elderly that our study found. These seizures are typically treated right away after an ischemic episode.
As previously noted by other researchers, the most prevalent type of seizure is tonic-clonic [21]. According to other studies, the prevalence of tonic-clonic epilepsy is 40%, which is in line with findings from Finland and Sweden (38% and 41%, respectively) [22, 23]. Due to clinical errors and the lack of paraclinical data, such as brain imaging results, half of the epilepsies that were first diagnosed as generalized are now categorized as secondary generalized epilepsy [20]. The higher frequency of epilepsy in males with an overall sex ratio of 1.5 may also be explained by the increased prevalence of symptomatic epilepsy in men, which is mostly attributable to head injury.
As previously stated, both the general population and those over 60 (the elderly) have higher rates of epileptic seizures. Furthermore, research from several nations indicates that older adults are more likely to experience their first epileptic seizure [24–26]. According to earlier research, seizures occur differently in young and old individuals. Younger people are more likely to experience generalized seizures, whereas older people are more likely to experience focal seizures [27]. According to our study, generalized seizures are more common in younger persons than in adults over 60, which is consistent with other studies [11, 28, 29]. However, individuals aged > 60 years are more likely to experience subsequent generalized and mixed seizures [22]. According to earlier research, the majority of seizures in young people originate in the temporal lobes; however, seizures that originate outside of the temporal lobes are more common in older individuals [8, 11, 23]. The different anatomical causes of seizures in these two age groups account for the observed discrepancy.
MRI findings in the study participants showed that the most common finding was Gliosis and Encephalomalacia in 27 patients. This suggests a strong correlation between these anomalies and epilepsy symptoms in the population under study. Identifying these recurring trends could impact the diagnosis of epilepsy and help comprehend its underlying neurobiological causes.
We compared our results with those of previous studies to provide context. The relationship between epilepsy and MRI results has been the subject of numerous studies, and our findings are consistent with some of the prevalent patterns. For example, gliosis and encephalomalacia anomalies were highly prevalent in the groups of patients with epilepsy studied by Sillanpää et al. [30] and Brodie et al. [26]. It is crucial to remember that variations in sample size, demographics, and imaging procedures can cause disparities in research findings. Different abnormalities may be highlighted in different research, or differing prevalence rates may be reported.
We contribute to the ongoing discussion in this area and provide a more comprehensive understanding of the various MRI findings associated with epilepsy by integrating our findings with the existing literature [19, 26, 31]. This comparative analysis contributes to the body of knowledge in this field and opens the door to better methods of diagnosis and treatment for patients with epilepsy.
According to our research, the most prevalent subtype of epilepsy was tonic-clonic epilepsy, which involved 160 individuals, whereas 37 patients had focal epilepsy without unconsciousness. These results clarify the distribution of epilepsy subtypes in the study sample. We compared our findings with those of previous studies on the prevalence of epilepsy subgroups to corroborate our results. Our research supports several earlier studies that consistently identified tonic-clonic epilepsy as one of the most prevalent subtypes [23, 25, 32]. Due to its unique and identifiable characteristics, which frequently result in a timely diagnosis, tonic-clonic epilepsy is very common. Our study’s prevalence of focal epilepsy without loss of consciousness, however, seems to be slightly lower than that reported in earlier studies. Differences in study populations, geographic regions, and diagnostic criteria may have caused this disparity. The symptoms of focal epilepsy without loss of consciousness are frequently mild and transient, making precise diagnosis difficult and frequently missed by many friends.
Conclusion
According to this study, tonic-clonic and focal epilepsy were the most prevalent forms of epilepsy in Ardabil County among adults. Interestingly, fewer focal instances were found than anticipated. Thus, it is essential to do more accurate MRIs, repeat and extend EEGs, and inform caregivers and medical professionals about focal seizure symptoms, particularly in elderly individuals. This method may result in a more precise diagnosis of seizure types and, as a result, more successful treatments. In addition to improving healthcare services for surgery or other treatments in drug-resistant epilepsy, these data can help estimate the target population for novel AEDs.
Limitations of the study
Our study had several limitations, which are outlined as follows: It was a single-center retrospective study. There was no precise information on the regular use of prescribed medications by patients. This study did not include long-term follow-up (beyond one year). Additionally, in most cases, the type of seizure was not observed by the treating physicians.
Strengths of the study
This study’s strengths include its large, representative sample and comprehensive data collection, covering key demographic and clinical factors, such as comorbidities and risk factors. The use of standardized questionnaires and consistent clinical evaluations ensured the reliability of the results. Additionally, focusing on MRI and EEG findings provides deeper insights into the neurological aspects of epilepsy. These strengths make the study valuable for healthcare providers and policymakers, aiding in better understanding and management of epilepsy in the Ardabil region
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research Ethics Committee of Ardabil University of Medical Sciences (Code: IR.ARUMS.REC.1401.134).
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
We extend our sincere gratitude to the staff and administration of Alavi Hospital in Ardabil city, Iran, for their assistance and support during the data collection phase of this study. We are also grateful to the patients and their families for their participation and cooperation. Additionally, we acknowledge the contributions of Ardabil University of Medical Sciences for providing ethical approval and necessary resources to conduct this research.
References
- Betjemann JP, Lowenstein DH. Status epilepticus in adults. The Lancet. Neurology. 2015; 14(6):615-24. [DOI:10.1016/S1474-4422(15)00042-3] [PMID]
- Roberg LE, Monsson O, Kristensen SB, Dahl SM, Ulvin LB, Heuser K, et al. Prediction of long-term survival after status epilepticus using the ACD Score. JAMA Neurology. 2022; 79(6):604-13. [DOI:10.1001/jamaneurol.2022.0609] [PMID]
- Schubert-Bast S, Zöllner JP, Ansorge S, Hapfelmeier J, Bonthapally V, Eldar-Lissai A, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: A population-based study on German health insurance data. Epilepsia. 2019; 60(5):911-20. [DOI:10.1111/epi.14729] [PMID]
- Poukas VS, Pollard JR, Anderson CT. Rescue therapies for seizures. Current Neurology and Neuroscience Reports. 2011; 11(4):418-22. [DOI:10.1007/s11910-011-0207-x] [PMID]
- Humphries LK, Eiland LS. Treatment of acute seizures: Is intranasal midazolam a viable option? The Journal of Pediatric Pharmacology and Therapeutics : JPPT : The Official Journal of PPAG. 2013; 18(2):79-87. [DOI:10.5863/1551-6776-18.2.79] [PMID]
- Anderson GD, Saneto RP. Current oral and non-oral routes of antiepileptic drug delivery. Advanced Drug Delivery Reviews. 2012; 64(10):911-8. [DOI:10.1016/j.addr.2012.01.017] [PMID]
- Kadel J, Bauer S, Hermsen AM, Immisch I, Kay L, Klein KM, et al. Use of emergency medication in adult patients with epilepsy: A multicentre cohort study from Germany. CNS Drugs. 2018; 32(8):771-81. [DOI:10.1007/s40263-018-0544-2] [PMID]
- Lathers CM, Jim KF, Spivey WH. A comparison of intraosseous and intravenous routes of administration for antiseizure agents. Epilepsia. 1989; 30(4):472-9.[DOI:10.1111/j.1528-1157.1989.tb05328.x] [PMID]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors.The Journal of Neuroscience. 1997; 17(19):7532-40. [DOI:10.1523/JNEUROSCI.17-19-07532.1997] [PMID]
- Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Archives of Pediatrics & Adolescent Medicine. 2010; 164(8):747-53.[DOI:10.1001/archpediatrics.2010.130] [PMID]
- Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999; 40(1):120-2. [DOI:10.1111/j.1528-1157.1999.tb02000.x] [PMID]
- Rosenow F, Besser R, Hamer HM, Holtkamp M, Kluge S, Knake S. [Status epilepticus im Erwachsenenalter. Leitlinien für Diagnostik und Therapie in der Neurologie (German)]. Stuttgart: Thieme Verlag; 2012. [Link]
- Strzelczyk A, Ansorge S, Hapfelmeier J, Bonthapally V, Erder MH, Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: A population-based study from Germany. Epilepsia. 2017; 58(9):1533-41. [DOI:10.1111/epi.13837] [PMID]
- Neligan A, Noyce AJ, Gosavi TD, Shorvon SD, Köhler S, Walker MC. Change in mortality of generalized convulsive status epilepticus in high-income countries over time: A systematic review and meta-analysis. JAMA Neurology. 2019; 76(8):897-905. [DOI:10.1001/jamaneurol.2019.1268] [PMID]
- Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993; 43(3 Pt 1):483-8. [DOI:10.1212/WNL.43.3_Part_1.483] [PMID]
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. The New England Journal of Medicine. 2001; 345(9):631-7. [DOI:10.1056/NEJMoa002141] [PMID]
- Sathe AG, Underwood E, Coles LD, Elm JJ, Silbergleit R, Chamberlain JM, et al. Patterns of benzodiazepine underdosing in the Established Status Epilepticus Treatment Trial. Epilepsia. 2021; 62(3):795-806. [DOI:10.1111/epi.16825] [PMID]
- Kellinghaus C, Rossetti AO, Trinka E, Lang N, May TW, Unterberger I, et al. Factors predicting cessation of status epilepticus in clinical practice: Data from a prospective observational registry (SENSE). Annals of Neurology. 2019; 85(3):421-32. [DOI:10.1002/ana.25416] [PMID]
- Willems LM, Bauer S, Jahnke K, Voss M, Rosenow F, Strzelczyk A. Therapeutic options for patients with refractory status epilepticus in palliative settings or with a limitation of life-sustaining therapies: A systematic review. CNS Drugs. 2020; 34(8):801-26. [DOI:10.1007/s40263-020-00747-z] [PMID]
- Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology. 2005; 65(8):1313-5. [DOI:10.1212/01.wnl.0000180685.84547.7f] [PMID]
- Herzog AG, Frye CA; Progesterone Trial Study Group. Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology. 2014 J; 83(4):345-8. [DOI:10.1212/WNL.0000000000000623] [PMID]
- Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM, Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. The New England Journal of Medicine. 1998; 338(26):1869-75. [DOI:10.1056/NEJM199806253382602] [PMID]
- Cereghino JJ, Mitchell WG, Murphy J, Kriel RL, Rosenfeld WE, Trevathan E. Treating repetitive seizures with a rectal diazepam formulation :A randomized study. The North American Diastat Study Group. Neurology. 1998; 51(5):1274-82. [DOI:10.1212/WNL.51.5.1274] [PMID]
- Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus--Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015; 56(10):1515-23. [DOI:10.1111/epi.13121] [PMID]
- Zarrelli MM, Beghi E, Rocca WA, Hauser WA. Incidence of epileptic syndromes in Rochester, Minnesota: 1980-1984. Epilepsia. 1999; 40(12):1708-14. [DOI:10.1111/j.1528-1157.1999.tb01587.x] [PMID]
- Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012; 78(20):1548-54. [DOI:10.1212/WNL.0b013e3182563b19] [PMID]
- Rose AB, McCabe PH, Gilliam FG, Smith BJ, Boggs JG, Ficker DM, et al. Occurrence of seizure clusters and status epilepticus during inpatient video-EEG monitoring. Neurology. 2003; 60(6):975-8. [DOI:10.1212/01.WNL.0000053748.83309.28] [PMID]
- Wirrell EC, Grossardt BR, Wong-Kisiel LC, Nickels KC. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: A population-based study. Epilepsy Research. 2011; 95(1-2):110-8. [DOI:10.1016/j.eplepsyres.2011.03.009] [PMID]
- Beghi E, Giussani G, Sander JW. The natural history and prognosis of epilepsy. Epileptic Disorders : International Epilepsy Journal with Videotape. 2015; 17(3):243-53. [DOI:10.1684/epd.2015.0751] [PMID]
- Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: Prospective, long-term population-based study. Brain. 2006; 129(Pt 3):617-24. [DOI:10.1093/brain/awh726] [PMID]
- Annegers JF, Hauser WA, Elveback LR. Remission of seizures and relapse in patients with epilepsy. Epilepsia. 1979; 20(6):729-37. [DOI:10.1111/j.1528-1157.1979.tb04857.x] [PMID]
- Berg AT, Rychlik K. The course of childhood-onset epilepsy over the first two decades: A prospective, longitudinal study. Epilepsia. 2015; 56(1):40-8. [DOI:10.1111/epi.12862] [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/09/22 | Accepted: 2024/12/31 | Published: 2025/10/18
Received: 2024/09/22 | Accepted: 2024/12/31 | Published: 2025/10/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






