Thu, Jan 29, 2026
Volume 15, Issue 4 (Autumn 2025)
PTJ 2025, 15(4): 275-288 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azadian E, Majlesi M, Siahvashi M, DehghanNasab A. Gait Variability During Obstacle Crossing in Children With Intellectual Disabilities. PTJ 2025; 15 (4) :275-288
URL: http://ptj.uswr.ac.ir/article-1-668-en.html
URL: http://ptj.uswr.ac.ir/article-1-668-en.html
1- Department of Motor Behavior, Faculty of Humanities, Ha.C., Islamic Azad University, Hamedan, Iran.
2- Department of Sport Biomechanics, Faculty of Humanities, Ha.C., Islamic Azad University, Hamedan, Iran.
2- Department of Sport Biomechanics, Faculty of Humanities, Ha.C., Islamic Azad University, Hamedan, Iran.
Keywords: Intellectual disability, Gait, Motor control, Obstacle crossing, Spatio-temporal gait parameters
Full-Text [PDF 1128 kb]
(400 Downloads)
| Abstract (HTML) (1174 Views)
Full-Text: (300 Views)
Introduction
Intellectual disability (ID), affecting approximately 1% of the population, is characterized as “a condition originating during the developmental phase, marked by limitations in intellectual abilities and adaptive, social, and practical skills” [1]. Although this definition does not directly relate to the physical and motor performance of individuals with ID, the examination of motor functions in this group has recently become a topic of great interest.
ID can result from various genetic and environmental influences, resulting in a highly diverse population [2]. In cases where ID is linked to a genetic syndrome, such as Down syndrome (DS), the presence of specific physical characteristics (e.g. ligamentous laxity and muscular hypotonia) leads to weaknesses in both static and dynamic balance, increased variability in postural control [3, 4], and delayed attainment of gross motor skills [5]. Nonetheless, motor performance is impacted even in children and adults with ID who do not have genetic causes. For instance, the average age at which walking begins is notably delayed compared to individuals with typical development (TD) [6, 7]. Some studies have also emphasized that impairments in movement-related cognitive functions, such as executive functions [8], sensory systems [9], and underdevelopment of the central nervous system [10], in children with ID result in weaker balance [11], immature postural control, and an increased risk and frequency of falls compared to TD children [12].
Evaluating gait in children with ID is rarely conducted due to the challenges these individuals face in understanding instructions. Several studies have indicated that children with ID demonstrate reduced step lengths, slower walking speeds, and shorter single-leg stance times compared to children with TD [13, 14]. In contrast, a study by Sparrow et al. reported higher walking speeds and cadence, but shorter step lengths and step times in the ID group [15]. The examination of step-to-step variability in these variables has mainly focused on adolescents and adults with DS, with results indicating greater variability in gait parameters compared to control groups [16, 17]. The primary aim of this study is to examine the spatial-temporal gait characteristics and their variability in children with ID and to compare these findings with those of age-matched children with TD.
Efficient movement involves maintaining a rhythmic movement pattern, controlling dynamic body balance, adapting to environmental changes, and achieving task goals [18-20]. Navigating an obstacle along a path necessitates the coordination of various perceptual, cognitive, and motor functions, including attention, planning, and memory. This integration poses a significant challenge to preserving motor performance [21]. Adaptive movements necessitate a harmonic combination of the sensory and motor systems. Sensory and motor integration occurs at three control levels: 1) Designing the motor program, which requires perception and cognition, 2) Assessing the environment and accurately planning the movement path, and 3) Adapting and modifying speed and direction as necessary to stay on course [22]. Motor adjustments and adaptability are strategies that lead to behavioral changes enabling the achievement of the final goal and ensuring successful motor performance [23]. In walking and obstacle crossing, modifying certain movement characteristics, such as speed, can reduce the risk of collision or falling [14, 24]. Numerous studies have investigated the effects of obstacle crossing on the kinematic and kinetic gait characteristics in adults and young individuals with DS [25]. Reports indicate that encountering obstacles during DS results in motor adjustments such as a reduction in the percentage of the stance phase, decreased speed and step length, increased step width [26], and greater variability [27]. Children with ID also exhibit an initial quick movement followed by a decrease in speed when crossing obstacles [15].
Examining gait characteristics, especially under challenging conditions, in children with ID who have lower cognitive abilities, is a suitable method for identifying adaptive problems and issues related to gait control, which have received less attention from researchers. Consequently, this study aimed to explore the impact of obstacle crossing on gait characteristics and the variability of spatial and temporal parameters in children with ID and to compare these characteristics with those of TD children.
Materials and Methods
This study used a descriptive comparative research design. The sample size was estimated using G*Power software, with a power of 80%, an effect size of 0.25, and α=0.05 [28], requiring a minimum of 30 participants across the two groups. The study included 16 girls aged 8-13 with educable and mild ID (IQ: 60-70) without genetic disorders (based on reports from doctors, parents, and teachers documented in school records), selected purposefully from elementary special education schools. The control group comprised 17 children with typical intelligence, conveniently selected from elementary and middle school. Due to the influence of maturation during this developmental stage, the control group was carefully paired with the ID group based on age and sex.
In this study, ID was defined based on the World Health Organization’s (WHO) 1st Edition of the International Classification of Diseases and the Spanish National Government guidelines (Royal Order 1971/1999, December 23). Participants had a disability score exceeding 30%, determined by combining their intelligence quotient and adaptive behavior scores. These scores were categorized into five ascending levels: Non-existent (0%), limited (15–29%), mild (30–59%), moderate (60–75%), and severe or very severe (76%). Therefore, the inclusion criteria were age 8-13 for both groups and mild to moderate ID for the ID group. The exclusion criteria for all children included the presence of neurological disorders (excluding intellectual disability for the ID group), long-term health conditions, substantial visual deficits, and physical disabilities that could impact gait. The study aims and assessment methods were explained to the parents, and they signed consent forms for their children. Data were collected in the morning in the presence of parents or teachers. The researchers had been present at the ID group’s school for at least a month prior, and the participants were thoroughly familiar with them. Therefore, in the laboratory, the only challenge for these children was becoming familiar with the laboratory environment, and they were given enough time and guidance to learn the testing procedures.
Instrumentation and procedures
A Vicon motion analysis system, equipped with six T-series cameras operating at 100 Hz, and two force plates (Kistler type 9281, Kistler Instrument AG, Winterthur, Switzerland) recording at 1000 Hz, was utilized to capture three-dimensional kinematic data. This setup measured spatiotemporal parameters and identified gait events during barefoot walking on a flat surface. Sixteen spherical reflective markers were strategically placed to define the pelvis, thighs, legs, and feet according to the Plug-in Gait model [29]. Participants were allowed to acclimate to the laboratory setting before the tests were administrated. A minimum of ten minutes of instruction was provided for each test [30]. The participants were instructed to walk at a self-selected speed along the walkway and step over the force plates [31]. Six trials were conducted with a one-minute break between each, and three successful trials were selected for further analysis [32].
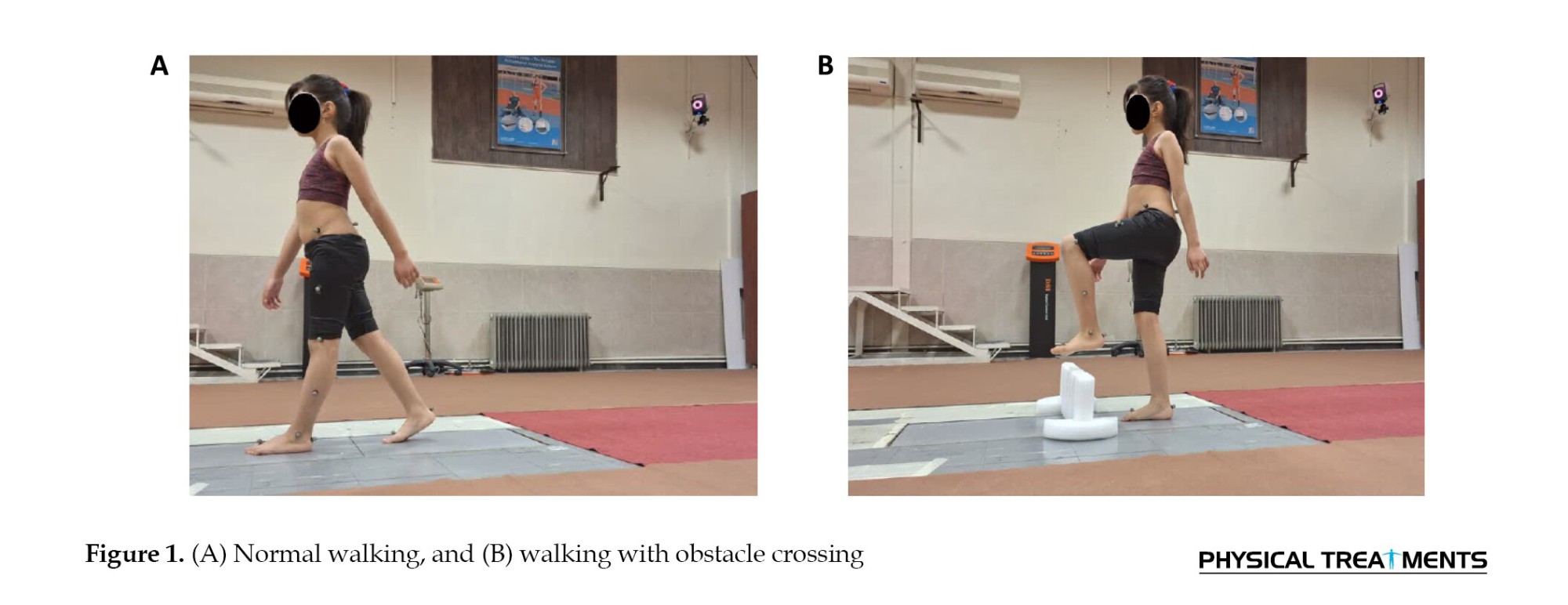
In this study, participants were assigned two tasks: (A) Normal walking, and (B) Walking with obstacle crossing. In this study, the obstacle was a flexible foam material measuring 15 cm in height, 60 cm in width, and 6 cm in depth, placed at the center of the calibrated area (Figure 1) [33].
Likewise, the kinematic data were processed using a zero-lag, fourth-order low-pass Butterworth filter with a cut-off frequency of 6 Hz. Nexus software (Vicon Motion Systems, Oxford, UK) was utilized to synchronize the kinematic and ground reaction force data. The spatiotemporal gait parameters and their variability were analyzed during normal walking and walking while crossing an obstacle. Considering the impact of participants’ height on spatiotemporal gait parameters, and given the significant inter-group difference in height, all gait variables were normalized to account for height. To estimate the step-to-step variability for each parameter, the Mean±SD of three consecutive steps were calculated, and the coefficient of variation (CoV) was computed using the Equation 1 [34].
1. CoV=(SD/mean)×100
Statistical analyses
The normality of the outcome measures was evaluated using the Shapiro-Wilk test. Descriptive statistics were calculated for both demographic and outcome variables. Since the balance variables followed a normal distribution, parametric methods were applied for analysis. To compare demographic data between the two groups, an independent t-test was employed. Analysis of variance (ANOVA) was used to examine the differences between the two groups in spatial-temporal parameter data. Considering the presence of two within-subject factors, Task factors included normal walking and walking while crossing an obstacle, and a leg factor (leading and trailing legs). A between-subject factor (ID and control groups) was also included. A three-way repeated measures ANOVA was employed to investigate the effects of these factors. Data were analyzed using SPSS software, version 21, with statistical significance set at P<0.05.
Results
Table 1 presents the participants’ demographic characteristics. The analysis revealed significant differences between the two groups in terms of height, weight, leg length, and ankle circumference (P<0.05).
Spatiotemporal gait parameters
The results of between-group comparisons indicated that the ID group had significantly lower gait speed during obstacle crossing (OBS) task and shorter stride and step lengths than the TD group in both tasks (Figure 2).
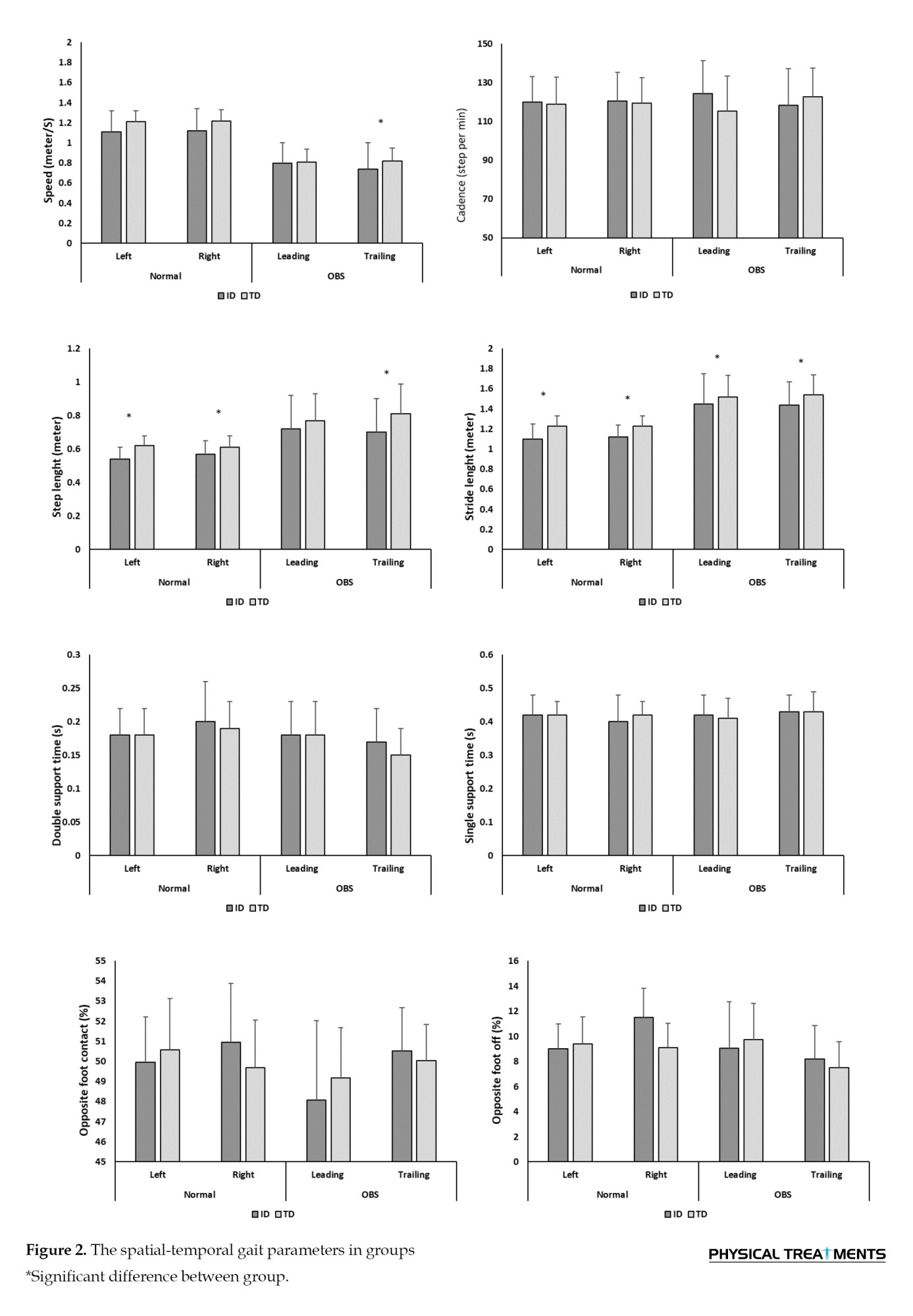
The results showed that the main effect of task had no significant effect on spatial variables (speed, cadence, step length, and stride length). As shown in Table 2, the main effect of foot and the interaction between the foot and task factors were significant for cadence. Additionally, the interaction between foot and task was significant for walking speed. The paired-wise comparison revealed that, during the obstacle-crossing task, the trailing leg had lower speed and cadence compared to normal walking (P<0.05).
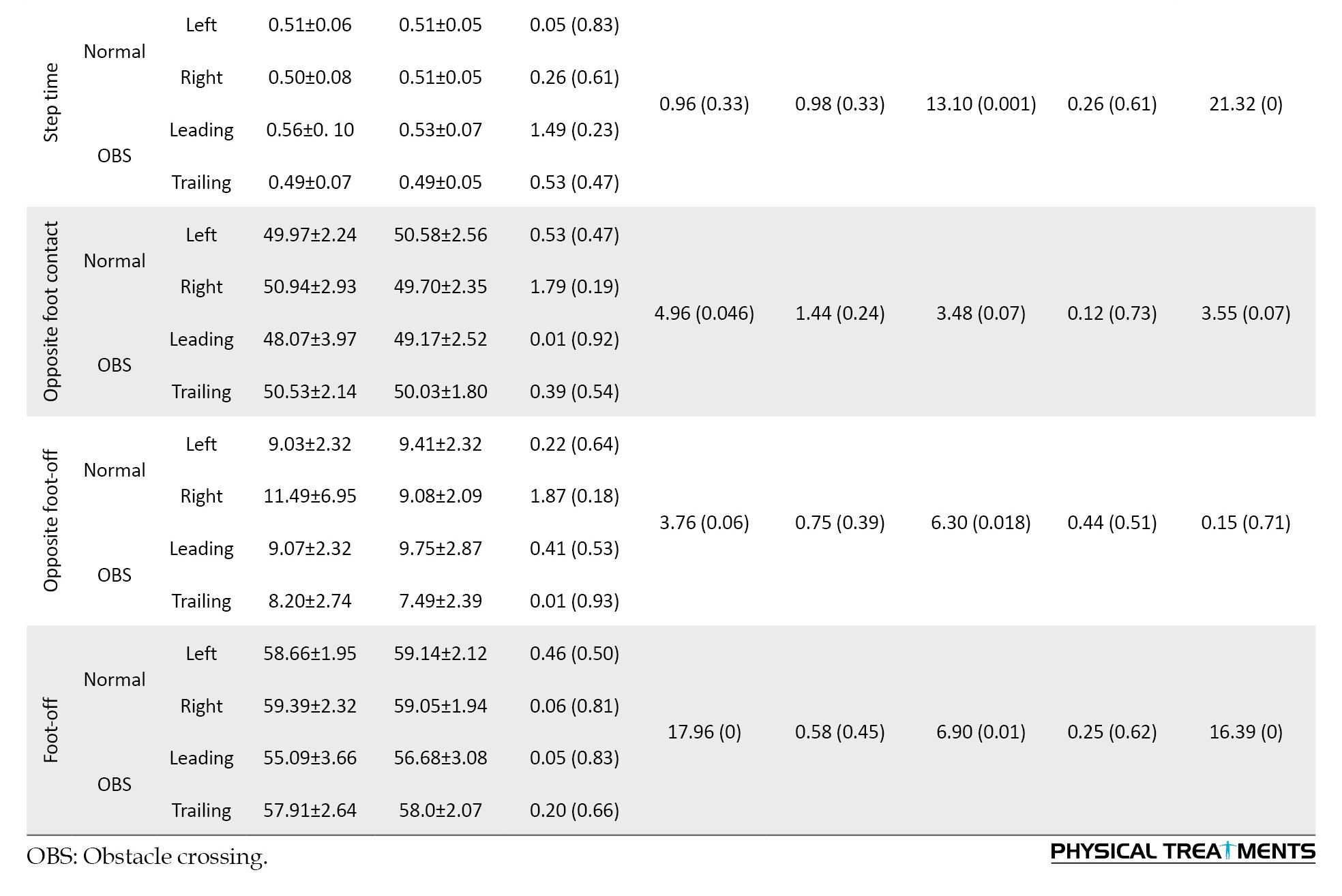
In light of the results from the temporal parameters, the main effect of the task and foot had a significant effect on double support time (P<0.05). The paired-wise comparison indicated that during OBS walking, the double-support time in the trailing leg decreased by approximately 11%. For the step and stride time variables, the foot factor and the interaction between the foot and task factors were significant (P<0.05). These results show that OBS led to a reduction in step and stride times compared to normal walking, especially in the trailing leg (Table 2).
The factor analysis results showed that the main effect of task had significant effects on opposite foot-contact and foot-off (P<0.05). Also, the main effect of the foot had significant effects on the opposite foot-off and foot-off (P<0.05). The mean comparison indicated that these percentages were significantly lower in the obstacle task than in normal walking, and the percentage of foot-off occurred significantly earlier in the leading leg (P<0.05).
Variability in spatiotemporal gait parameters
According to the between-group results, the variability in most variables was greater in the ID group than in the TD group. The between-group results, as shown in Table 3, demonstrated the variability in speed, stride, and step length, double support time, and percentage parameters during normal gait, and cadence, stride, and step time in the trailing leg during OBS gait. Speed was significantly higher in the ID group than the TD group (P<0.05).
However, the task factor did not show a significant effect on the variability of the spatial and temporal gait parameters (P>0.05). The interaction between group and task in double support time and opposite foot-off showed that the variability in the obstacle-crossing task was lower in the ID group compared to the normal condition, whereas, in the TD group, the variability in the obstacle-crossing condition was higher than in the normal condition (P<0.05).
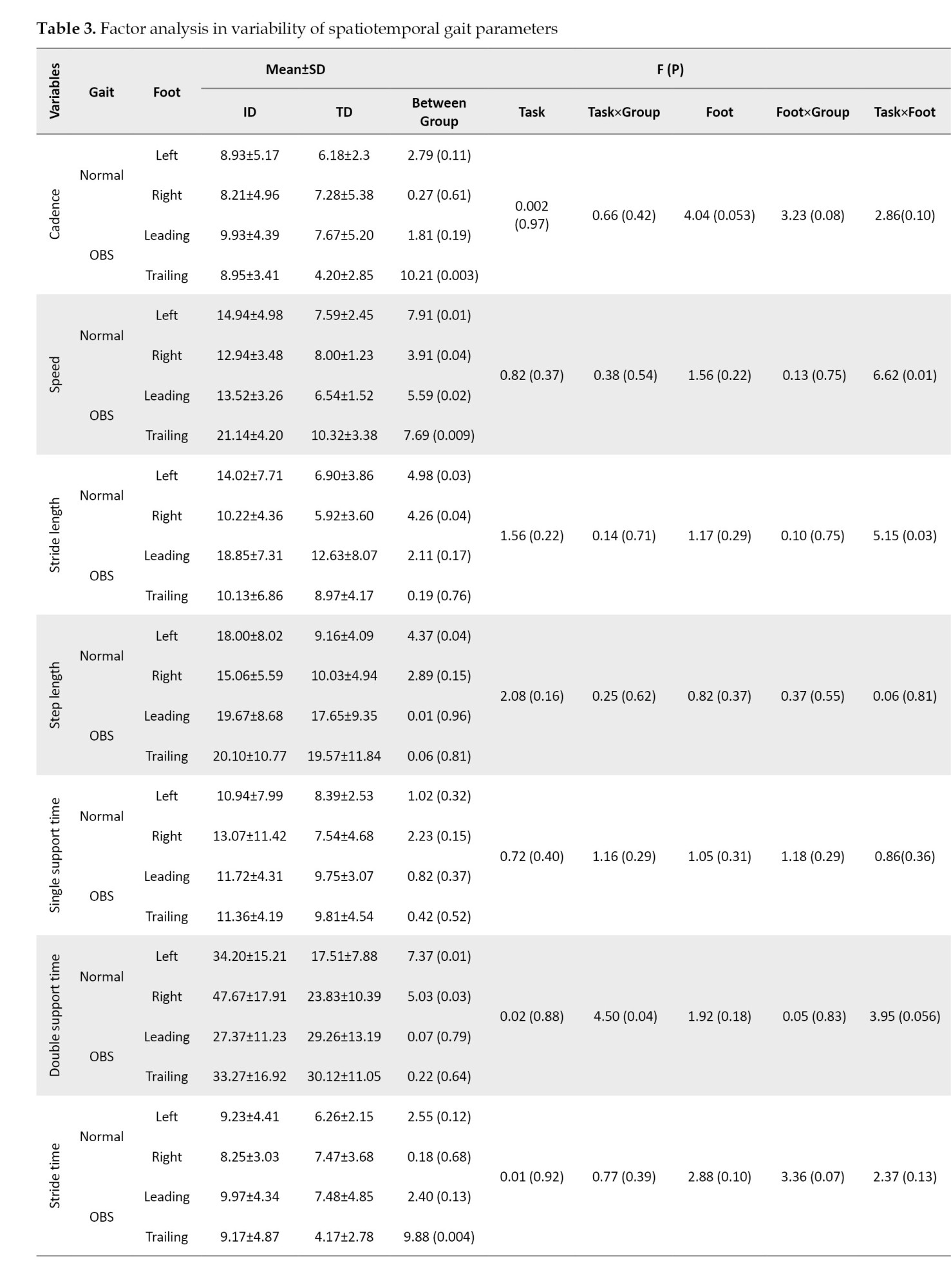
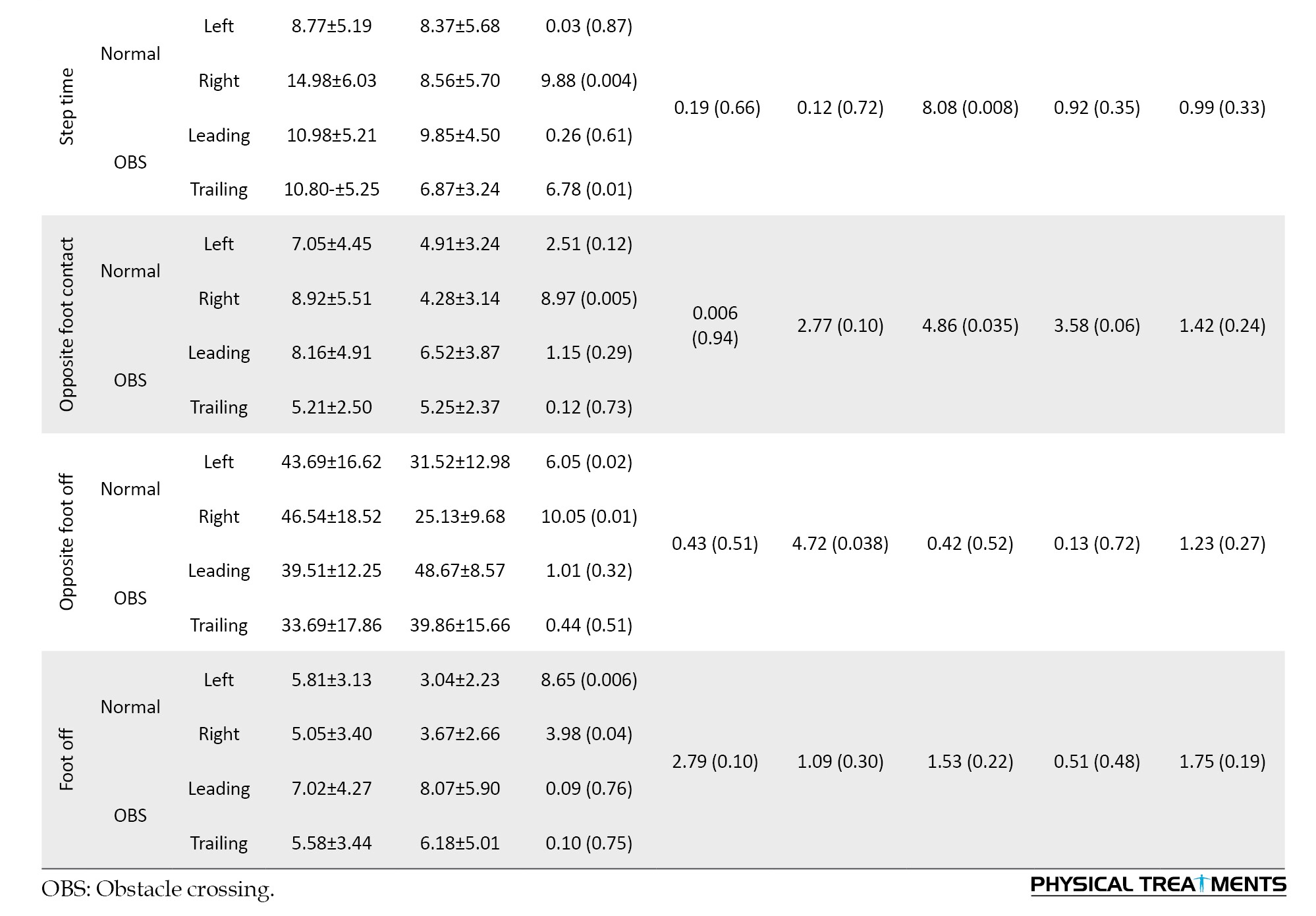
Furthermore, the interaction between task and foot was significant for speed walking and step length. Table 3 shows that the variability in these variables, especially in the ID group, increased significantly during the trailing leg of obstacle crossing compared to other conditions (P<0.05).
Discussion
This study was conducted to assess the variability of gait and obstacle crossing in children with ID and compare it with that in TD children of TD. As shown in the results, the stride and step lengths in the ID group were lower than those in the TD group during normal walking. The results of the few studies conducted on individuals with ID without genetic disorders have shown that these individuals have lower cadence [11], step length [11, 13, 14, 35], and walking speeds [13, 35] compared to their TD peers. However, some studies have reported higher walking speeds and cadences, but lower step lengths and step times, in men and women with ID compared to their TD counterparts [15]. Based on these findings, the decrease in step and stride length could be attributed to the restricted range of motion in the joints of these individuals. Cameron et al. demonstrated that agonist and antagonist muscles are not well coordinated in individuals with ID [36]. Consequently, abnormal muscle tension during the swing phase of walking leads to joint stiffness and a reduced range of motion in the lower limbs [18]. However, to draw more comprehensive conclusions, additional gait variables in individuals with ID must be examined.
However, walking while obstacle crossing compared to normal walking led to a decrease in double support time in the trailing leg and a reduction in stance percentage in the leading leg in both groups. Additionally, in the ID group, the speed and number of cadences in the obstacle-crossing task were lower in the trailing leg compared to normal walking. Previous studies have shown that successful obstacle crossing requires timely detachment and appropriate toe clearance [37]. The present study showed that both groups reduced the stance time of the leading leg by approximately 8% during obstacle crossing, which consequently reduced the double support time in the trailing leg. Few studies have used a challenging task, such as obstacle crossing to study gait in individuals with ID, with most participants in these studies being adults with DS. The results of these studies have indicated that the most significant difference between individuals with DS and the control group is their performance before crossing the obstacle [11]. In individuals with DS, multiple stops were observed before crossing an obstacle, indicating the need for more time to plan and execute the movement [26]. Another study reported that the ID group showed quick obstacle crossing, with an 80% reduction in the gait cycle time of the leading leg, followed by a decrease in speed [15], and decreased sway time in the trailing leg [38]. Thus, the reduction in walking speed observed in participants of this study, especially in the trailing leg, aligns with these results and indicates caution to avoid obstacles for individuals with ID.
According to the results, the variability in most spatial and temporal gait parameters was significantly higher in the ID group compared to the control group. Variability in cadence, speed, double support time, step time, and stride time was significantly higher in the ID group than in the TD group. Similar studies have shown that step length in individuals with DS follows a more irregular pattern [17], and that variability in gait patterns is higher in these individuals compared to their TD peers [27]. Our study also found that the amount of variability in children with ID was significantly higher than in the control group, which can be interpreted in two ways: 1) The traditional interpretation explains higher variability as a limitation, indicating immature motor control, posture, and the nervous system, ultimately increasing the risk of falls [39]; 2) The dynamic systems approach suggests that increased variability may indicate a strategy for adapting to environmental change. Variability in motor execution is a characteristic of the nervous system, representing the degree of adaptability and maturity of motor control. It depends on individual characteristics, environmental constraints, and tasks [40]. According to Stergiou et al., the amount of variability and disorder follows an inverted U-shape, meaning that with a severe reduction, movements become entirely predictable, and the individual has minimal adaptability to environmental changes [41, 42]. Illness usually leads to decreased variability, indicating joint locking and reduced degrees of freedom [41]. However, when disorder increases significantly, individuals become vulnerable to small environmental changes. Thus, an optimal amount of entropy or variability, appropriate to individual characteristics, enhances an individual’s adaptability to the environment [43].
In this study, given the significant differences in speed and stride length parameters between the two groups, and the higher variability in the ID group, this may indicate a strategy for adapting to new conditions, such as obstacle crossing. Based on the results regarding the interaction between task and group, the variability in double support time and opposite foot-off in the ID group decreased during normal walking compared to the TD group. Double support time is an indicator of balance and stability during walking. Previous studies have shown that individuals with ID have poorer postural control and balance outcomes compared to typically developing individuals [44-46]. According to the reduction in variability of this parameter, it seems that individuals with ID reduce degrees of freedom in their joints, resulting in less adaptability to environmental changes [47] and maintaining safety during walking.
In contrast, the trailing leg speed in the ID group decreased during obstacle crossing, along with a significant increase in variability. Vimercati et al. showed that individuals with DS tend to land closer to the obstacle when crossing it with their head and trailing foot [27]. Consistent with the findings of the present study, they also demonstrated that the variability in the trailing foot during obstacle crossing is higher in individuals with DS compared to those with TD, which could indicate a risk of hitting the obstacle with the trailing leg [27]. Previous research also aligns with this study, showing that the lack of visual information when crossing the trailing leg over the obstacle increases the likelihood of hitting the obstacle by 27% [48]. Thus, despite reducing trailing leg speed during obstacle crossing in the ID group, increased variability in this leg may indicate a lack of precise control in the absence of vision, thereby increasing the risk of hitting obstacle and falling [49].
Conclusion
According to the results of this study, spatial and temporal parameters in both normal and obstacle walking conditions were similar in children with ID and the TD group. Given the similarity of most spatial and temporal gait parameters in the ID group, increased step-to-step variability in this group may be a strategy for adapting to the environment. However, the decreased speed of the trailing leg during obstacle crossing, along with increased variability in the speed of this leg in individuals with, indicates reduced motor control and adaptability, consequently increasing the risk of hitting the obstacle. Based on these results, practicing any skill or ability, such as walking in the ID group, may lead to improved performance of that skill.
One of the limitations in this study was the exclusion of boys due to their non-compliance with the inclusion criteria, such as age, the presence of secondary sensory, neurological, or motor disorders, which led to their exclusion from the study. Also, the lack of homogenization of individuals with ID based on fundamental abilities reduces the generalizability of this study. Therefore, it is recommended to use both genders in future studies to investigate movement characteristics.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Islamic Azad University, Hamadan Branch, Hamadan, Iran (Code: IR.IAU.H.REC.1402.008), and complied with the ethical principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments. Additional ethical considerations included ensuring participants’ freedom to engage in the study, guaranteeing the confidentiality of the information, and maintaining anonymity. Before participating in the study, participants provided their informed consent after discussing the study objectives.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are deeply grateful to the participants and their families for their invaluable participation and support throughout this study.
References
Intellectual disability (ID), affecting approximately 1% of the population, is characterized as “a condition originating during the developmental phase, marked by limitations in intellectual abilities and adaptive, social, and practical skills” [1]. Although this definition does not directly relate to the physical and motor performance of individuals with ID, the examination of motor functions in this group has recently become a topic of great interest.
ID can result from various genetic and environmental influences, resulting in a highly diverse population [2]. In cases where ID is linked to a genetic syndrome, such as Down syndrome (DS), the presence of specific physical characteristics (e.g. ligamentous laxity and muscular hypotonia) leads to weaknesses in both static and dynamic balance, increased variability in postural control [3, 4], and delayed attainment of gross motor skills [5]. Nonetheless, motor performance is impacted even in children and adults with ID who do not have genetic causes. For instance, the average age at which walking begins is notably delayed compared to individuals with typical development (TD) [6, 7]. Some studies have also emphasized that impairments in movement-related cognitive functions, such as executive functions [8], sensory systems [9], and underdevelopment of the central nervous system [10], in children with ID result in weaker balance [11], immature postural control, and an increased risk and frequency of falls compared to TD children [12].
Evaluating gait in children with ID is rarely conducted due to the challenges these individuals face in understanding instructions. Several studies have indicated that children with ID demonstrate reduced step lengths, slower walking speeds, and shorter single-leg stance times compared to children with TD [13, 14]. In contrast, a study by Sparrow et al. reported higher walking speeds and cadence, but shorter step lengths and step times in the ID group [15]. The examination of step-to-step variability in these variables has mainly focused on adolescents and adults with DS, with results indicating greater variability in gait parameters compared to control groups [16, 17]. The primary aim of this study is to examine the spatial-temporal gait characteristics and their variability in children with ID and to compare these findings with those of age-matched children with TD.
Efficient movement involves maintaining a rhythmic movement pattern, controlling dynamic body balance, adapting to environmental changes, and achieving task goals [18-20]. Navigating an obstacle along a path necessitates the coordination of various perceptual, cognitive, and motor functions, including attention, planning, and memory. This integration poses a significant challenge to preserving motor performance [21]. Adaptive movements necessitate a harmonic combination of the sensory and motor systems. Sensory and motor integration occurs at three control levels: 1) Designing the motor program, which requires perception and cognition, 2) Assessing the environment and accurately planning the movement path, and 3) Adapting and modifying speed and direction as necessary to stay on course [22]. Motor adjustments and adaptability are strategies that lead to behavioral changes enabling the achievement of the final goal and ensuring successful motor performance [23]. In walking and obstacle crossing, modifying certain movement characteristics, such as speed, can reduce the risk of collision or falling [14, 24]. Numerous studies have investigated the effects of obstacle crossing on the kinematic and kinetic gait characteristics in adults and young individuals with DS [25]. Reports indicate that encountering obstacles during DS results in motor adjustments such as a reduction in the percentage of the stance phase, decreased speed and step length, increased step width [26], and greater variability [27]. Children with ID also exhibit an initial quick movement followed by a decrease in speed when crossing obstacles [15].
Examining gait characteristics, especially under challenging conditions, in children with ID who have lower cognitive abilities, is a suitable method for identifying adaptive problems and issues related to gait control, which have received less attention from researchers. Consequently, this study aimed to explore the impact of obstacle crossing on gait characteristics and the variability of spatial and temporal parameters in children with ID and to compare these characteristics with those of TD children.
Materials and Methods
This study used a descriptive comparative research design. The sample size was estimated using G*Power software, with a power of 80%, an effect size of 0.25, and α=0.05 [28], requiring a minimum of 30 participants across the two groups. The study included 16 girls aged 8-13 with educable and mild ID (IQ: 60-70) without genetic disorders (based on reports from doctors, parents, and teachers documented in school records), selected purposefully from elementary special education schools. The control group comprised 17 children with typical intelligence, conveniently selected from elementary and middle school. Due to the influence of maturation during this developmental stage, the control group was carefully paired with the ID group based on age and sex.
In this study, ID was defined based on the World Health Organization’s (WHO) 1st Edition of the International Classification of Diseases and the Spanish National Government guidelines (Royal Order 1971/1999, December 23). Participants had a disability score exceeding 30%, determined by combining their intelligence quotient and adaptive behavior scores. These scores were categorized into five ascending levels: Non-existent (0%), limited (15–29%), mild (30–59%), moderate (60–75%), and severe or very severe (76%). Therefore, the inclusion criteria were age 8-13 for both groups and mild to moderate ID for the ID group. The exclusion criteria for all children included the presence of neurological disorders (excluding intellectual disability for the ID group), long-term health conditions, substantial visual deficits, and physical disabilities that could impact gait. The study aims and assessment methods were explained to the parents, and they signed consent forms for their children. Data were collected in the morning in the presence of parents or teachers. The researchers had been present at the ID group’s school for at least a month prior, and the participants were thoroughly familiar with them. Therefore, in the laboratory, the only challenge for these children was becoming familiar with the laboratory environment, and they were given enough time and guidance to learn the testing procedures.
Instrumentation and procedures
A Vicon motion analysis system, equipped with six T-series cameras operating at 100 Hz, and two force plates (Kistler type 9281, Kistler Instrument AG, Winterthur, Switzerland) recording at 1000 Hz, was utilized to capture three-dimensional kinematic data. This setup measured spatiotemporal parameters and identified gait events during barefoot walking on a flat surface. Sixteen spherical reflective markers were strategically placed to define the pelvis, thighs, legs, and feet according to the Plug-in Gait model [29]. Participants were allowed to acclimate to the laboratory setting before the tests were administrated. A minimum of ten minutes of instruction was provided for each test [30]. The participants were instructed to walk at a self-selected speed along the walkway and step over the force plates [31]. Six trials were conducted with a one-minute break between each, and three successful trials were selected for further analysis [32].
In this study, participants were assigned two tasks: (A) Normal walking, and (B) Walking with obstacle crossing. In this study, the obstacle was a flexible foam material measuring 15 cm in height, 60 cm in width, and 6 cm in depth, placed at the center of the calibrated area (Figure 1) [33].

Likewise, the kinematic data were processed using a zero-lag, fourth-order low-pass Butterworth filter with a cut-off frequency of 6 Hz. Nexus software (Vicon Motion Systems, Oxford, UK) was utilized to synchronize the kinematic and ground reaction force data. The spatiotemporal gait parameters and their variability were analyzed during normal walking and walking while crossing an obstacle. Considering the impact of participants’ height on spatiotemporal gait parameters, and given the significant inter-group difference in height, all gait variables were normalized to account for height. To estimate the step-to-step variability for each parameter, the Mean±SD of three consecutive steps were calculated, and the coefficient of variation (CoV) was computed using the Equation 1 [34].
1. CoV=(SD/mean)×100
Statistical analyses
The normality of the outcome measures was evaluated using the Shapiro-Wilk test. Descriptive statistics were calculated for both demographic and outcome variables. Since the balance variables followed a normal distribution, parametric methods were applied for analysis. To compare demographic data between the two groups, an independent t-test was employed. Analysis of variance (ANOVA) was used to examine the differences between the two groups in spatial-temporal parameter data. Considering the presence of two within-subject factors, Task factors included normal walking and walking while crossing an obstacle, and a leg factor (leading and trailing legs). A between-subject factor (ID and control groups) was also included. A three-way repeated measures ANOVA was employed to investigate the effects of these factors. Data were analyzed using SPSS software, version 21, with statistical significance set at P<0.05.
Results
Table 1 presents the participants’ demographic characteristics. The analysis revealed significant differences between the two groups in terms of height, weight, leg length, and ankle circumference (P<0.05).

Spatiotemporal gait parameters
The results of between-group comparisons indicated that the ID group had significantly lower gait speed during obstacle crossing (OBS) task and shorter stride and step lengths than the TD group in both tasks (Figure 2).

The results showed that the main effect of task had no significant effect on spatial variables (speed, cadence, step length, and stride length). As shown in Table 2, the main effect of foot and the interaction between the foot and task factors were significant for cadence. Additionally, the interaction between foot and task was significant for walking speed. The paired-wise comparison revealed that, during the obstacle-crossing task, the trailing leg had lower speed and cadence compared to normal walking (P<0.05).


In light of the results from the temporal parameters, the main effect of the task and foot had a significant effect on double support time (P<0.05). The paired-wise comparison indicated that during OBS walking, the double-support time in the trailing leg decreased by approximately 11%. For the step and stride time variables, the foot factor and the interaction between the foot and task factors were significant (P<0.05). These results show that OBS led to a reduction in step and stride times compared to normal walking, especially in the trailing leg (Table 2).
The factor analysis results showed that the main effect of task had significant effects on opposite foot-contact and foot-off (P<0.05). Also, the main effect of the foot had significant effects on the opposite foot-off and foot-off (P<0.05). The mean comparison indicated that these percentages were significantly lower in the obstacle task than in normal walking, and the percentage of foot-off occurred significantly earlier in the leading leg (P<0.05).
Variability in spatiotemporal gait parameters
According to the between-group results, the variability in most variables was greater in the ID group than in the TD group. The between-group results, as shown in Table 3, demonstrated the variability in speed, stride, and step length, double support time, and percentage parameters during normal gait, and cadence, stride, and step time in the trailing leg during OBS gait. Speed was significantly higher in the ID group than the TD group (P<0.05).
However, the task factor did not show a significant effect on the variability of the spatial and temporal gait parameters (P>0.05). The interaction between group and task in double support time and opposite foot-off showed that the variability in the obstacle-crossing task was lower in the ID group compared to the normal condition, whereas, in the TD group, the variability in the obstacle-crossing condition was higher than in the normal condition (P<0.05).


Furthermore, the interaction between task and foot was significant for speed walking and step length. Table 3 shows that the variability in these variables, especially in the ID group, increased significantly during the trailing leg of obstacle crossing compared to other conditions (P<0.05).
Discussion
This study was conducted to assess the variability of gait and obstacle crossing in children with ID and compare it with that in TD children of TD. As shown in the results, the stride and step lengths in the ID group were lower than those in the TD group during normal walking. The results of the few studies conducted on individuals with ID without genetic disorders have shown that these individuals have lower cadence [11], step length [11, 13, 14, 35], and walking speeds [13, 35] compared to their TD peers. However, some studies have reported higher walking speeds and cadences, but lower step lengths and step times, in men and women with ID compared to their TD counterparts [15]. Based on these findings, the decrease in step and stride length could be attributed to the restricted range of motion in the joints of these individuals. Cameron et al. demonstrated that agonist and antagonist muscles are not well coordinated in individuals with ID [36]. Consequently, abnormal muscle tension during the swing phase of walking leads to joint stiffness and a reduced range of motion in the lower limbs [18]. However, to draw more comprehensive conclusions, additional gait variables in individuals with ID must be examined.
However, walking while obstacle crossing compared to normal walking led to a decrease in double support time in the trailing leg and a reduction in stance percentage in the leading leg in both groups. Additionally, in the ID group, the speed and number of cadences in the obstacle-crossing task were lower in the trailing leg compared to normal walking. Previous studies have shown that successful obstacle crossing requires timely detachment and appropriate toe clearance [37]. The present study showed that both groups reduced the stance time of the leading leg by approximately 8% during obstacle crossing, which consequently reduced the double support time in the trailing leg. Few studies have used a challenging task, such as obstacle crossing to study gait in individuals with ID, with most participants in these studies being adults with DS. The results of these studies have indicated that the most significant difference between individuals with DS and the control group is their performance before crossing the obstacle [11]. In individuals with DS, multiple stops were observed before crossing an obstacle, indicating the need for more time to plan and execute the movement [26]. Another study reported that the ID group showed quick obstacle crossing, with an 80% reduction in the gait cycle time of the leading leg, followed by a decrease in speed [15], and decreased sway time in the trailing leg [38]. Thus, the reduction in walking speed observed in participants of this study, especially in the trailing leg, aligns with these results and indicates caution to avoid obstacles for individuals with ID.
According to the results, the variability in most spatial and temporal gait parameters was significantly higher in the ID group compared to the control group. Variability in cadence, speed, double support time, step time, and stride time was significantly higher in the ID group than in the TD group. Similar studies have shown that step length in individuals with DS follows a more irregular pattern [17], and that variability in gait patterns is higher in these individuals compared to their TD peers [27]. Our study also found that the amount of variability in children with ID was significantly higher than in the control group, which can be interpreted in two ways: 1) The traditional interpretation explains higher variability as a limitation, indicating immature motor control, posture, and the nervous system, ultimately increasing the risk of falls [39]; 2) The dynamic systems approach suggests that increased variability may indicate a strategy for adapting to environmental change. Variability in motor execution is a characteristic of the nervous system, representing the degree of adaptability and maturity of motor control. It depends on individual characteristics, environmental constraints, and tasks [40]. According to Stergiou et al., the amount of variability and disorder follows an inverted U-shape, meaning that with a severe reduction, movements become entirely predictable, and the individual has minimal adaptability to environmental changes [41, 42]. Illness usually leads to decreased variability, indicating joint locking and reduced degrees of freedom [41]. However, when disorder increases significantly, individuals become vulnerable to small environmental changes. Thus, an optimal amount of entropy or variability, appropriate to individual characteristics, enhances an individual’s adaptability to the environment [43].
In this study, given the significant differences in speed and stride length parameters between the two groups, and the higher variability in the ID group, this may indicate a strategy for adapting to new conditions, such as obstacle crossing. Based on the results regarding the interaction between task and group, the variability in double support time and opposite foot-off in the ID group decreased during normal walking compared to the TD group. Double support time is an indicator of balance and stability during walking. Previous studies have shown that individuals with ID have poorer postural control and balance outcomes compared to typically developing individuals [44-46]. According to the reduction in variability of this parameter, it seems that individuals with ID reduce degrees of freedom in their joints, resulting in less adaptability to environmental changes [47] and maintaining safety during walking.
In contrast, the trailing leg speed in the ID group decreased during obstacle crossing, along with a significant increase in variability. Vimercati et al. showed that individuals with DS tend to land closer to the obstacle when crossing it with their head and trailing foot [27]. Consistent with the findings of the present study, they also demonstrated that the variability in the trailing foot during obstacle crossing is higher in individuals with DS compared to those with TD, which could indicate a risk of hitting the obstacle with the trailing leg [27]. Previous research also aligns with this study, showing that the lack of visual information when crossing the trailing leg over the obstacle increases the likelihood of hitting the obstacle by 27% [48]. Thus, despite reducing trailing leg speed during obstacle crossing in the ID group, increased variability in this leg may indicate a lack of precise control in the absence of vision, thereby increasing the risk of hitting obstacle and falling [49].
Conclusion
According to the results of this study, spatial and temporal parameters in both normal and obstacle walking conditions were similar in children with ID and the TD group. Given the similarity of most spatial and temporal gait parameters in the ID group, increased step-to-step variability in this group may be a strategy for adapting to the environment. However, the decreased speed of the trailing leg during obstacle crossing, along with increased variability in the speed of this leg in individuals with, indicates reduced motor control and adaptability, consequently increasing the risk of hitting the obstacle. Based on these results, practicing any skill or ability, such as walking in the ID group, may lead to improved performance of that skill.
One of the limitations in this study was the exclusion of boys due to their non-compliance with the inclusion criteria, such as age, the presence of secondary sensory, neurological, or motor disorders, which led to their exclusion from the study. Also, the lack of homogenization of individuals with ID based on fundamental abilities reduces the generalizability of this study. Therefore, it is recommended to use both genders in future studies to investigate movement characteristics.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Islamic Azad University, Hamadan Branch, Hamadan, Iran (Code: IR.IAU.H.REC.1402.008), and complied with the ethical principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments. Additional ethical considerations included ensuring participants’ freedom to engage in the study, guaranteeing the confidentiality of the information, and maintaining anonymity. Before participating in the study, participants provided their informed consent after discussing the study objectives.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are deeply grateful to the participants and their families for their invaluable participation and support throughout this study.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR). Washington: American Psychiatric Association; 2000. [Link]
- Hudac CM, Friedman NR, Ward VR, Estreicher RE, Dorsey GC, Bernier RA, et al. Characterizing sensory phenotypes of subgroups with a known genetic etiology pertaining to diagnoses of autism spectrum disorder and intellectual disability. Journal of Autism and Developmental Disorders. 2024; 54(6):2386-401. [DOI:10.1007/s10803-023-05897-9] [PMID]
- Dehghan Nasab A, Azadian E. [Relationship between fundamental movement skills and variability in postural control: Comparison of children with and without intellectual disability (Persian)]. Pajouhan Scientific Journal. 2024; 22(1):31-41. [DOI:10.61186/psj.22.1.31]
- Ghobadi M, Naderi S, Azadian E. [The relationship between balance performance and working memory capacity in individuals with and without Down syndrome (Persian)]. The Scientific Journal of Rehabilitation Medicine. 2019; 8(2):129-37. [DOI:10.22037/jrm.2018.111433.1990]
- Pereira K, Basso RP, Lindquist AR, Da Silva LG, Tudella E. Infants with Down syndrome: Percentage and age for acquisition of gross motor skills. Research in Developmental Disabilities. 2013; 34(3):894-901. [DOI:10.1016/j.ridd.2012.11.021] [PMID]
- Bishop SL, Thurm A, Farmer C, Lord C. Autism spectrum disorder, intellectual disability, and delayed walking. Pediatrics. 2016; 137(3):e20152959. [DOI:10.1542/peds.2015-2959] [PMID]
- Smits-Engelsman B, Hill EL. The relationship between motor coordination and intelligence across the IQ range. Pediatrics. 2012; 130(4):e950-e6. [DOI:10.1542/peds.2011-3712] [PMID]
- Hartman E, Houwen S, Scherder E, Visscher C. On the relationship between motor performance and executive functioning in children with intellectual disabilities. Journal of Intellectual Disability Research. 2010; 54(5):468-77. [DOI:10.1111/j.1365-2788.2010.01284.x] [PMID]
- Lipowicz A, Bugdol MN, Szurmik T, Bibrowicz K, Kurzeja P, Mitas AW. Body balance analysis of children and youth with intellectual disabilities. Journal of Intellectual Disability Research. 2019; 63(11):1312-23. [DOI:10.1111/jir.12671] [PMID]
- Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, et al. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. Neuroimage. 2008; 40(4):1533-41.[DOI:10.1016/j.neuroimage.2008.01.063] [PMID]
- Enkelaar L, Smulders E, van Schrojenstein Lantman-de Valk H, Geurts AC, Weerdesteyn V. A review of balance and gait capacities in relation to falls in persons with intellectual disability. Research in Developmental Disabilities. 2012; 33(1):291-306. [DOI:10.1016/j.ridd.2011.08.028] [PMID]
- Kachouri H, Borji R, Baccouch R, Laatar R, Rebai H, Sahli S. The effect of a combined strength and proprioceptive training on muscle strength and postural balance in boys with intellectual disability: An exploratory study. Research in Developmental Disabilities. 2016; 53:367-76. [DOI:10.1016/j.ridd.2016.03.003] [PMID]
- Haynes CA, Lockhart TE. Evaluation of gait and slip parameters for adults with intellectual disability. Journal of Biomechanics. 2012; 45(14):2337-41. [DOI:10.1016/j.jbiomech.2012.07.003] [PMID]
- Ma Y, Zhang K, Li S, Wang L, Wang T. Biomechanical analysis of gait patterns in children with intellectual disabilities. Journal of Intellectual Disability Research. 2021; 65(10):912-21. [DOI:10.1111/jir.12872] [PMID]
- Sparrow W, Shinkfield AJ, Summers J. Gait characteristics in individuals with mental retardation: Unobstructed level-walking, negotiating obstacles, and stair climbing. Human Movement Science. 1998; 17(2):167-87. [DOI:10.1016/S0167-9457(97)00028-6]
- Cabrera-Linares JC, Latorre Román PÁ, Párraga Montilla JA, Andrade-Lara KE, Ruiz-Peralvarez FJ, Gutierrez-Cruz C. Effects of a dual-task activity on gait parameters of people with and without intellectual disabilities. Journal of Intellectual Disability Research. 2024; 68(6):610-9. [DOI:10.1111/jir.13134] [PMID]
- Smith BA, Stergiou N, Ulrich BD. Patterns of gait variability across the lifespan in persons with and without down syndrome. Journal of Neurologic Physical Therapy. 2011; 35(4):170-7. [DOI:10.1097/NPT.0b013e3182386de1] [PMID]
- Almuhtaseb S, Oppewal A, Hilgenkamp TI. Gait characteristics in individuals with intellectual disabilities: A literature review. Research in Developmental Disabilities. 2014; 35(11):2858-83. [DOI:10.1016/j.ridd.2014.07.017] [PMID]
- Vuijk PJ, Hartman E, Scherder E, Visscher C. Motor performance of children with mild intellectual disability and borderline intellectual functioning. Journal of Intellectual Disability Research. 2010; 54(11):955-65. [DOI:10.1111/j.1365-2788.2010.01318.x] [PMID]
- Wuang YP, Wang CC, Huang MH, Su CY. Profiles and cognitive predictors of motor functions among early school-age children with mild intellectual disabilities. Journal of Intellectual Disability Research. 2008; 52(12):1048-60. [DOI:10.1111/j.1365-2788.2008.01096.x] [PMID]
- Hallemans A, Van de Walle P, Wyers L, Verheyen K, Schoonjans A, Desloovere K, et al. Clinical usefulness and challenges of instrumented motion analysis in patients with intellectual disabilities. Gait & Posture. 2019; 71:105-15. [DOI:10.1016/j.gaitpost.2019.04.016] [PMID]
- da Silva JJ, Barbieri FA, Gobbi L TB. Adaptive locomotion for crossing a moving obstacle. Motor Control. 2011; 15(3):419-33. [DOI:10.1123/mcj.15.3.419] [PMID]
- Klotzbier TJ, Bühler K, Holfelder B, Schott N. Exploring motor-cognitive interference in children with Down syndrome using the Trail-Walking-Test. Research in Developmental Disabilities. 2020; 106:103769. [DOI:10.1016/j.ridd.2020.103769] [PMID]
- Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, et al. Gait, gait variability and the risk of multiple incident falls in older people: A population-based study. Age and Ageing. 2011; 40(4):481-7. [DOI:10.1093/ageing/afr055] [PMID]
- Vali Noghondar N, Saberi Kakhki A, Sohrabi M, Alirezaei Noghondar F. Variability and coordination patterns of walking with different speeds in active and non-active children with Down syndrome: A cross-sectional case-control study. International Journal of Developmental Disabilities. 2021; 68(5):723-31. [DOI:10.1080/20473869.2021.1893923] [PMID]
- Smith BA, Ulrich BD. Early onset of stabilizing strategies for gait and obstacles: Older adults with Down syndrome. Gait & Posture. 2008; 28(3):448-55. [DOI:10.1016/j.gaitpost.2008.02.002] [PMID]
- Vimercati SL, Galli M, Rigoldi C, Albertini G. Obstacle avoidance in Down syndrome. Journal of Electromyography and Kinesiology. 2013; 23(2):483-9. [DOI:10.1016/j.jelekin.2012.10.006] [PMID]
- Lakens D. Sample size justification. Collabra: Psychology. 2022; 8(1):33267. [DOI:10.1525/collabra.33267]
- Azadian E, Majlesi M, Jafarnezhadgero AA, Granacher U. The impact of hearing loss on three-dimensional lower limb joint torques during walking in prepubertal boys. Journal of Bodywork and Movement Therapies. 2020; 24(2):123-9. [DOI:10.1016/j.jbmt.2019.10.013] [PMID]
- Lockhart TE, Woldstad JC, Smith JL. Effects of age-related gait changes on the biomechanics of slips and falls. Ergonomics. 2003; 46(12):1136-60. [DOI:10.1080/0014013031000139491] [PMID]
- Chardon M, Barbieri FA, Petit P, Vuillerme N. Reliability of obstacle-crossing parameters during overground walking in young adults. Sensors. 2024; 24(11):3387. [DOI:10.3390/s24113387] [PMID]
- Azadian E, Dadgar SA, Majlesi M, Jafarnezhadgero AA, Jalilvand M, Bijarchian MH. The effects of cognitive intervention on inter-joint coordination during walking in the older adult with balance impairment. Gait & Posture. 2023; 106:72-9. [DOI:10.1016/j.gaitpost.2023.08.030] [PMID]
- Ambike S, Penedo T, Kulkarni A, Santinelli FB, Barbieri FA. Step length synergy while crossing obstacles is weaker in patients with Parkinson’s disease. Gait & Posture. 2021; 84:340-5. [DOI:10.1016/j.gaitpost.2021.01.002] [PMID]
- Hocking DR, Menant JC, Kirk HE, Lord S, Porter MA. Gait profiles as indicators of domain-specific impairments in executive control across neurodevelopmental disorders. Research in Developmental Disabilities. 2014; 35(1):203-14. [DOI:10.1016/j.ridd.2013.10.005] [PMID]
- Chiba Y, Shimada A, Yoshida F, Keino H, Hasegawa M, Ikari H, et al. Risk of fall for individuals with intellectual disability. American Journal on Intellectual and Developmental Disabilities. 2009; 114(4):225-36. [DOI:10.1352/1944-7558-114.4:225-236] [PMID]
- Cameron KL, Albesher RA, McGinley JL, Allison K, Cheong JLY, Spittle AJ. Movement-based interventions for preschool-age children with, or at risk of, motor impairment: A systematic review. Developmental Medicine and Child Neurology. 2020; 62(3):290-6. [DOI:10.1111/dmcn.14394] [PMID]
- Raffegeau TE, Brinkerhoff SA, Kellaher GK, Baudendistel S, Terza MJ, Roper JA, et al. Changes to margins of stability from walking to obstacle crossing in older adults while walking fast and with a dual-task. Experimental Gerontology. 2022; 161:111710. [DOI:10.1016/j.exger.2022.111710] [PMID]
- Lu SH, Kuan YC, Wu KW, Lu HY, Tsai YL, Chen HH, et al. Kinematic strategies for obstacle-crossing in older adults with mild cognitive impairment. Frontiers in Aging Neuroscience. 2022; 14:950411. [DOI:10.3389/fnagi.2022.950411] [PMID]
- Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiology of Aging. 2002; 23(1):1-11. [DOI:10.1016/S0197-4580(01)00247-0] [PMID]
- Pierce SR, Paremski AC, Skorup J, Stergiou N, Senderling B, Prosser LA. Linear and nonlinear measures of postural control in a toddler with cerebral palsy: Brief report. Pediatric Physical Therapy. 2020; 32(1):80-3. [DOI:10.1097/PEP.0000000000000669] [PMID]
- Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Human Movement Science. 2011; 30(5):869-88. [DOI:10.1016/j.humov.2011.06.002] [PMID]
- Stergiou N, Yu Y, Kyvelidou A. A perspective on human movement variability with applications in infancy motor development. Kinesiology Review. 2013; 2(1):93-102. [DOI:10.1123/krj.2.1.93]
- Lipsitz LA, Goldberger AL. Loss of’complexity’and aging: Potential applications of fractals and chaos theory to senescence. JAMA. 1992; 267(13):1806-9. [DOI:10.1001/jama.1992.03480130122036]
- Chen HL, Yeh CF, Howe TH. Postural control during standing reach in children with Down syndrome. Research in Developmental Disabilities. 2015; 38:345-51. [DOI:10.1016/j.ridd.2014.12.024] [PMID]
- Wang HY, Long IM, Liu MF. Relationships between task-oriented postural control and motor ability in children and adolescents with Down syndrome. Research in Developmental Disabilities. 2012; 33(6):1792-8. [DOI:10.1016/j.ridd.2012.05.002] [PMID]
- Habibi Masouleh Z, Shamsi Majalani A, Sedaghati P. [Comparing the postural control among different age ranges in intellectually disabled girls (Persian)]. Journal of Sport Biomechanics. 2021; 6(4):240-9. [DOI:10.32598/biomechanics.6.3.3]
- Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Developmental Psychobiology. 2003; 42(4):368-77. [DOI:10.1002/dev.10110] [PMID]
- Diaz GJ, Parade MS, Barton SL, Fajen BR. The pickup of visual information about size and location during approach to an obstacle. PLoS One. 2018; 13(2):e0192044. [DOI:10.1371/journal.pone.0192044] [PMID]
- Doherty AJ, Benedetto V, Harris C, Ridley J, O'Donoghue A, James-Jenkinson L, et al. Preventing falls at home among people with intellectual disabilities: A scoping review. Journal of Applied Research in Intellectual Disabilities. 2023; 36(4):702-24. [DOI:10.1111/jar.13104] [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/08/9 | Accepted: 2024/12/30 | Published: 2025/10/18
Received: 2024/08/9 | Accepted: 2024/12/30 | Published: 2025/10/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






