Fri, Sep 19, 2025
Volume 15, Issue 3 (Summer 2025)
PTJ 2025, 15(3): 205-216 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haghighi A, Ebrahimi M, Askari R, Nouri G, khademosharie M. The Effects of Two Different Intensities of Concurrent Training on Pulmonary Function in Female Patients With Type II Diabetes. PTJ 2025; 15 (3) :205-216
URL: http://ptj.uswr.ac.ir/article-1-661-en.html
URL: http://ptj.uswr.ac.ir/article-1-661-en.html
Amirhossein Haghighi *1 

 , Malihe Ebrahimi1
, Malihe Ebrahimi1 
 , Roya Askari1
, Roya Askari1 

 , Gholamreza Nouri2
, Gholamreza Nouri2 
 , Mitra Khademosharie3
, Mitra Khademosharie3 




 , Malihe Ebrahimi1
, Malihe Ebrahimi1 
 , Roya Askari1
, Roya Askari1 

 , Gholamreza Nouri2
, Gholamreza Nouri2 
 , Mitra Khademosharie3
, Mitra Khademosharie3 


1- Department of Exercise Physiology, Faculty of Sport Sciences, Hakim Sabzevari University, Sabzevar, Iran.
2- Vasei Hospital, Sabzevari University of Medical Sicences, Sabzevar, Iran.
3- Department of Sport Sciences, Faculty of Humanities, Kosar University of Bojnord, Bojnord, Iran.
2- Vasei Hospital, Sabzevari University of Medical Sicences, Sabzevar, Iran.
3- Department of Sport Sciences, Faculty of Humanities, Kosar University of Bojnord, Bojnord, Iran.
Full-Text [PDF 522 kb]
(154 Downloads)
| Abstract (HTML) (963 Views)
Full-Text: (65 Views)
Introduction
Diabetes mellitus is a metabolic disorder that causes a lack of insulin production, a deficiency in its action, or both, resulting in hyperglycemia with impaired carbohydrate, lipid, and protein metabolism. The disease is chronic, serious, and progressive, with multiple damages to various organs. Major and long-term complications of diabetes include cardiovascular disease, diabetic nephropathy, diabetic retinopathy, and damage to other organs, such as the lungs. All of these result from macrovascular and microvascular damage [1].
The association between decreased lung function and diabetes has been described for many years, although the clinical significance of this association is unknown [2, 3]. Potential links between respiratory disorders and diabetes may be attributed to factors, such as elevated body mass index, reduced respiratory efficiency, neuropathy, weakened respiratory muscle strength, and other contributing variables [2]. Additionally, biochemical abnormalities, including inadequate glucose regulation, play a critical role in elevating the risk of pulmonary complications and related health conditions in individuals with diabetes [4, 5].
Pathological studies have shown that the causes of pulmonary dysfunction in patients with diabetes are the major changes in lung tissues, such as changes in the thickness of the alveolar wall, the thickness of the alveolar capillaries, and the lining thickness of the arterioles. Researchers have identified the main causes of these respiratory disorders as hyperglycemia and glycosylation of the chest wall muscles [6]. Some other studies have shown that increased respiratory infections, inflammation, and oxidative stress induced by diabetes reduce respiratory muscle function and limit pulmonary function [7-9]. The Women’s cardiovascular health research center of Britannia showed that insulin resistance and diabetes are associated with reductions in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) [10].
Various studies have suggested that physical activities can be effective in the prevention, control, and treatment of diabetes. Regular moderate exercise is effective in preventing and delaying the onset of diabetes mellitus (DM), increases insulin sensitivity, and improves glucose metabolism [11, 12]. Moreover, exercise training has been considered a therapeutic intervention in the pulmonary rehabilitation program of patients with pulmonary dysfunction [13, 14]. However, the type, severity, and duration of these exercises need further investigation. The American College of Sports Medicine and the American Diabetes Association have confirmed that rehabilitation programs for patients with diabetes should combine aerobic and resistance exercises to maximize the beneficial effects of both types of exercise [15].
Combining aerobic exercise with resistance training offers greater benefits than either modality alone in reducing inflammatory markers, regulating blood sugar and insulin function, and improving cardiovascular disease risk factors [16-19]. Despite extensive research on cardiovascular complications and diabetic nephropathy, retinopathy, and neuropathy, reduced pulmonary function in diabetic patients has received comparatively less attention. Additionally, studies investigating the effects of concurrent exercises on pulmonary function are relatively scarce. For instance, Tunkamnerdthai et al. demonstrated that an 8-week push-up exercise program (three sessions per week) improved FEV1, FVC, and maximal voluntary ventilation (MVV) while reducing HbA1c levels, although the FEV1/FVC ratio remained unchanged [20]. Similarly, Osho et al. examined the effects of a 12-week aerobic-strength training program (with and without weights) on the pulmonary function of 60 patients with type 2 diabetes aged 40–75 years. Their findings revealed significant improvements in VO2 max, FEV1, and HbA1c levels, with FVC and FEV1 indices showing greater enhancement in concurrent training with weights compared to training without weights or the control group [21].
Kim et al. stated that exercise training intensity is one of the factors that affect the strength and resistance of respiratory muscles and pulmonary function [22]. Concurrent exercise programs can enhance respiratory muscle strength and endurance, combining aerobic and resistance training components. Aerobic exercises, such as cycling or walking improve oxygen exchange efficiency and lung capacity, while resistance training strengthens the muscles involved in breathing—beneficial for individuals with type 2 diabetes who may experience respiratory muscle weakness due to the disease or a sedentary lifestyle [23]. Additionally, regular exercise has been shown to improve glycemic control, reflected in lower HbA1c levels. Concurrent exercise programs are particularly effective as they combine the immediate blood sugar-lowering effects of aerobic exercise with the longer-term benefits of resistance training on insulin sensitivity [24]. However, the optimal intensity, frequency, and duration of such programs for individuals with type 2 diabetes remain under investigation. Tailoring these programs to an individual’s fitness level, comorbidities, and medication regimen is critical for safety and effectiveness. Further research is warranted to fully elucidate the effects of concurrent exercise programs on respiratory muscle function, body composition, glycemic control, and overall physical condition in this population. Such studies should also address unique considerations for this group, including hypoglycemia risk, medication interactions, and the influence of diet and other lifestyle factors.
However, the effect of intensity of a concurrent exercise program on respiratory muscles, improvement of body composition, HbA1c, and the physical condition of people with type 2 diabetes is not taken into account and needs further investigation. Therefore, this study was designed to investigate the effect of a concurrent exercise training (aerobic and resistance) program with different intensities (high and low) on the pulmonary function of women with type 2 diabetes.
Materials and Methods
Study sample
The research method was quasi-experimental with pre-test and post-test design. The statistical population included obese women with type 2 diabetes who attended the diabetes community in Sabzevar and had medical records in this medical center. Thirty-four individuals with a history of at least five years of diabetes were selected through availability sampling according to the inclusion criteria. The inclusion criteria were women aged 45-60 years (all subjects were evaluated by a specialist and confirmed to be menopausal); a body mass index (BMI) of 27 to 34 kg/m²; no other diseases (such as autoimmune disorders, respiratory diseases, liver disease, cardiac ischemia, kidney disease, chronic inflammatory diseases, thyroid diseases, stomach ulcers, and infection), a diagnosis of type 2 diabetes; fasting blood glucose levels of less than 180 mg/dl; a 2-hour glucose level of less than 250 mg/dl; no insulin injections; non-smokers; no participation in regular sports programs during the past six months; and the ability to attend the study for three months. Individuals who did not meet these conditions were excluded from the study. Subsequently, ten participants refused to continue their participation for personal reasons and due to disease progression. The remaining 24 subjects were randomly assigned to three groups: low-intensity exercise (8 individuals), high-intensity exercise (8 individuals), and a control group (8 individuals). Written informed consent was obtained from all participants.
Training program
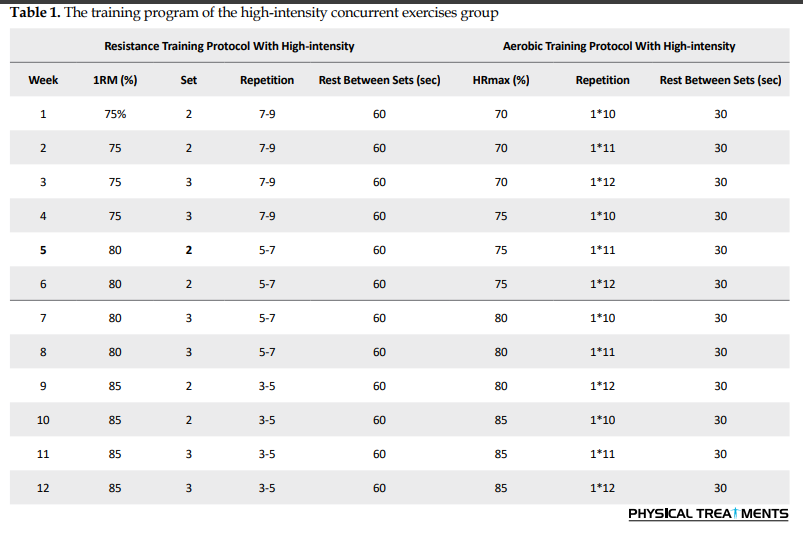
The study began at the sports physiology laboratory at Hakim Sabzevari University for anthropometric and physiological measurements on December 5, 2021. Height, weight, body fat percentage, and waist-hip ratio were measured one week before the beginning of the training program. The following two to three sessions were held in the practice hall to familiarize participants with the training conditions. The training intervention consisted of 12 weeks of concurrent exercise, combining resistance and aerobic activities, performed at two intensity levels: High intensity (resistance at 75–85% of 1-RM and aerobic at 70–85% of maximum heart rate) and low intensity (resistance at 50–75% of 1-RM and aerobic at 50–70% of maximum heart rate). Participants completed three nonconsecutive sessions per week, with each session lasting between 55 and 70 minutes.
The training program of high-intensity concurrent exercises group
The program consisted of 10 minutes of warm-up (jogging, hand and foot movements combined with stretching), followed by 30 minutes of strength training and 10 minutes of cool-down. The stations included bench presses, dumbbell bicep curls, triceps pushdowns, seated leg extensions, leg curls, butterfly exercises, and underhand cable pull-downs. The exercises were performed in accordance with Table 1. The rest interval between each station was set at 120 seconds. After a 15-20 minute rest, 20 minutes of aerobic running training was conducted, featuring 10-12 repetitions for 1 minute at 70-85% of the maximum heart rate, with 30 seconds of active rest between sets at 30-40% of the maximum heart rate (Table 1).
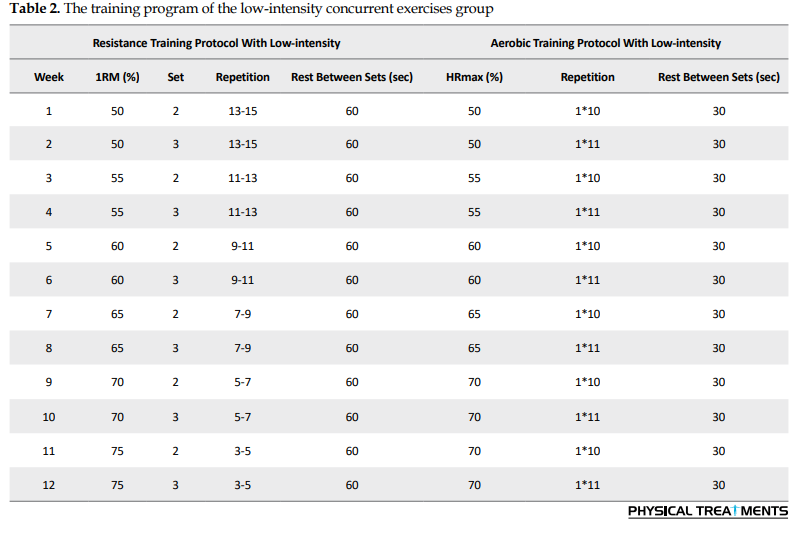
The training program of low-intensity concurrent exercises group
This program included 10 minutes of warm-up, 30 minutes of strength training exercises, and 10 minutes of cool-down. The rest interval between stations was considered also set at 120 seconds. After 20 minutes, aerobic running training at low intensity was performed according to Table 2.
Measurements
Blood samples were collected at two stages: Before and 24 hours after the last session, following a 10-12 hour fasting period. Glycosylated hemoglobin (HbA1c) was measured through an enzymatic colorimetric method with the Nicoard kit (made in Norway, catalog number 1042184), which has a coefficient of variation (CV) of less than 5%. To calculate the body fat percentage, a baseline caliper was used to measure subcutaneous fat in three areas: The thigh, upper pelvic area, and arm triceps, along with the Jackson and Pollock formulas [25]. Maximum oxygen consumption was measured using the Rockport 1-mile (1609 m) Walk Test [21, 26]. Indices of respiratory and pulmonary function were measured with a spirometer (model: CHESTGRAPH HI-701, France). The upper body strength was calculated using the bench press movement and the Brzycki Equation 1
1. 1RM=Weight÷(1.0278–(0.0278×number of repetitions)
Provided that the number of repetitions fell between six and ten repetitions. Upper body muscular strength was computed by the press movement and maximum repetitions to fatigue with 60% of 1RM [27, 28].
To measure the specific strength of the respiratory muscles, a spirometer was used [29]. After a full exhale, the subject was asked to place the mouthpiece in their mouth and breathe in sharply. As the subject inhaled, the piston moved upward, and the highest number displayed was considered the specific strength of the respiratory muscles. This test was repeated three times and the maximum value was calculated. At the same time, in order to monitor the participant’s diet, a 24-hour dietary recall questionnaire, and the three-day dietary records from the beginning and end of the study were used. Food data were analyzed using Nutrition software, version 4.
Statistical methods
Data were described as Mean±SD. The normality of the samples’ distribution was assessed using the Shapiro-Wilk test with Lilliefors correction for small samples, demonstrating a normal distribution for all samples. Treatment effects were analyzed using a two-way ANOVA (IBM SPSS software, version 21, Armonk, VA, USA). A significant interaction effect (group×time) indicated a treatment-dependent difference in the development from pre-test to post-test among the group conditions. Significance was accepted for P≤0.05.
Results
This study showed that 12 weeks of concurrent training at different intensities had significant therapeutic effects on some pulmonary variables, HbA1c values, and body fat percentage in women with type 2 diabetes. Table 3 shows the characteristics of the patients. Table 4 provides an overview of all physiological and functional variables, while Table 5 presents an overview of all respiratory variables.
.PNG)
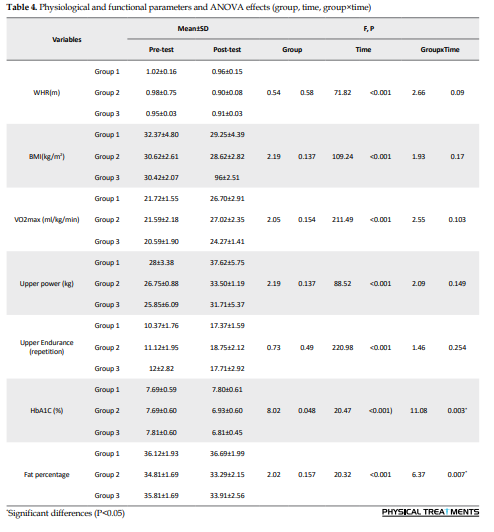
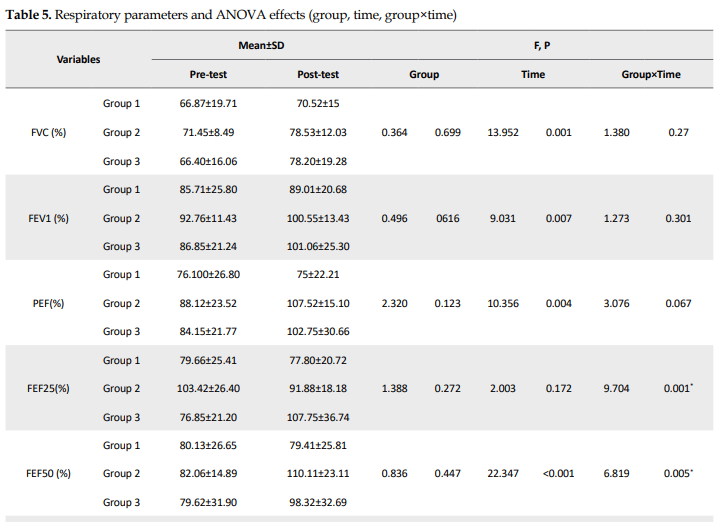
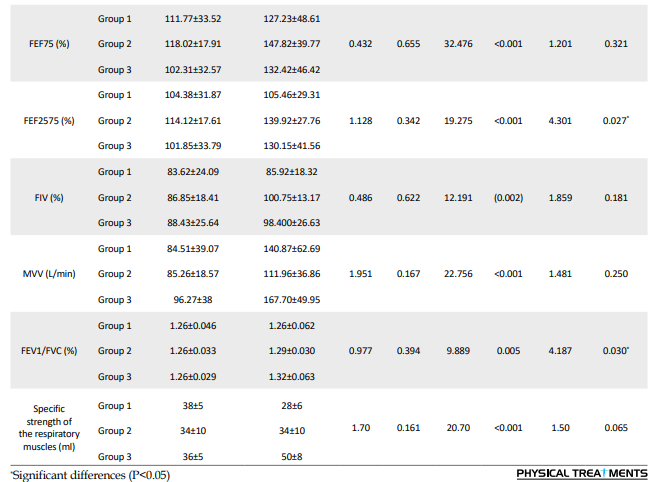
For several respiratory and physiological parameters, a significant interaction effect (group×time: P<0.05) was found, demonstrating a considerable influence of the respective treatments on the development from the pre-test to the post-test: FEF25, FEF50, FEF2575, FEV1/FVC, HbA1c, and fat percentage (Tables 4 and 5).
There were no significant group effects for other respiratory parameters (P>0.05). For some respiratory, physiological, and functional parameters, significant time effects were observed (P<0.05), indicating a significant change between the pre-test and post-test that was independent of the type of treatment: FVC, FEV, peak expiratory flow (PEF), FEF75, MVV, VO2max, upper body power, and upper body endurance (Tables 4 and 5).
Discussion
This study demonstrated that a 12-week concurrent training program with varying intensities had significant therapeutic effects on certain pulmonary variables, HbA1c levels, and body fat percentage in women with type 2 diabetes. These findings align with those of Silva-Reis et al. who reported that concurrent training enhances lung mechanics and reduces lung inflammation in overweight and obese women [30]. Similarly, Silva-Reis et al. found that concurrent exercise improved lung function, mechanics, and pulmonary immune responses in overweight and stage 1 obese women by elevating levels of the anti-fibrotic protein Klotho while reducing fibrotic IGF-1 [31]. These improvements were attributed to IGF-1, which is recognized as a primary growth factor overproduced in conditions, such as asthma and obesity. In asthma, IGF-1 plays a role in modulating airway inflammation, smooth muscle hyperresponsiveness, and hypertrophy, ultimately affecting lung mechanics [32]. Tunkamnerdthai et al. observed that an eight-week arm swing exercise program (three sessions per week) led to improvements in FEV1, FVC, and MVV while lowering HbA1c levels; however, the FEV1/FVC ratio remained unchanged in their study [20].
Osho et al. also documented significant enhancements in VO2max, FVC, FEV1, and HbA1c levels among 60 individuals with type 2 diabetes aged between 40 and 75 years [26]. Additionally, Chen et al. reported a notable increase in PEF following an eight-week kettlebell training program, indicating improved pulmonary function after exercise [33]. These findings suggest that the respiratory muscles of the participants in this study were effectively trained, resulting in significant improvements in PEF. While prior studies on resistance training in older adults have primarily examined its benefits for individuals with lung injuries or other specific conditions, fewer studies have focused on markers of lung function in this population [34].
Contrary to our findings, Jones and Nzekwu [35] reported no significant changes in lung volume. The results of our study can be explained by the acute enhancements in glucose metabolism that occur immediately after exercise, with these effects lasting for up to 48 hours, as noted by Davis et al. [36].
Poor glucose regulation is linked to microvascular complications and pulmonary respiratory distress [37]. Dyspnea is the leading cause of death in type 2 diabetes after adjusting for other known risk factors [38]. The reduction in mean HbA1c levels observed in this study, which aligns with findings from other research [37, 38] but contrasts with those reported by Sigal et al. [39], may contribute to significant improvements in FEV1/FVC values.
FVC is influenced by factors, such as age, physical activity level, body composition and health, respiratory muscle strength, and lung elasticity [40]. Additionally, the extent of lung volume reduction and airflow limitation correlates with glucose levels and body fat [41]. Hence, the increase in FVC observed in this study following concurrent training may be attributed to enhanced respiratory muscle strength and power, reduced body fat, and lower fasting blood glucose in patients with diabetes. However, the 12-week concurrent training program did not lead to a significant increase in FEV1 among women with type 2 diabetes in either training group. This outcome contrasts with findings from studies conducted by Osho et al. and Beckerman et al. [42]. FEV1, a specific measure of respiratory function, is affected by various factors. A decline in FEV1 indicates reduced lung capacity, airway obstruction, decreased lung reversibility, or, less commonly, insufficient respiratory muscle development. Thus, improving respiratory muscle strength can also enhance FEV1 [40]. As FEV1 serves as an indicator of respiratory muscle strength, it appears that exercises targeting the enhancement of respiratory muscle strength may contribute to increased FEV1 levels [43].
Many studies have reported reduced pulmonary function in diabetic patients, largely due to diminished strength and power of the respiratory muscles. In the present study, while upper and lower body muscle strength and power increased in both the low- and high-intensity training groups, these changes were not statistically significant. Although the strengthened muscles in this study primarily served as supporting muscles for pulmonary function rather than the primary muscles involved in respiration, their long-term strengthening appeared to contribute to improvements in respiratory system function. Additionally, aerobic training may enhance the diaphragm, intercostal, and abdominal muscles while reducing resistance in the respiratory tract. Notably, the specific strength of respiratory muscles, as measured by spirometry, improved in the low-intensity concurrent training group. This underscores the combined role of aerobic and resistance exercises in promoting pulmonary function. Prior research has also shown a positive and significant correlation between cardiovascular fitness (VO2max) and pulmonary function indices, such as FEV1 and FVC [44, 45].
The respiratory and cardiovascular systems collaborate closely to deliver oxygen to cells and regulate the internal environment of the body during both rest and activity. This partnership ensures a balance between ventilation and cardiac function in the gas exchange process that links skeletal muscles to atmospheric air. Any inefficiency in these systems can impair overall body function. When the ventilation-to-blood flow ratio or the ventilation-to-oxygen absorption and removal ratio declines relative to ventilation volume, respiratory muscles consume more energy, increasing the likelihood of premature fatigue [45]. Although the current study observed improvements in maximum oxygen consumption (VO2max) in both training groups, these changes were not statistically significant. Nevertheless, this factor may partially explain the observed enhancements in pulmonary function. This study demonstrated improvements across several key indicators, including respiratory muscle strength and power, body fat percentage, body weight, physical fitness, aerobic capacity, blood glucose levels, and HbA1c among the participants. The variability in results across studies may stem from differences in participant characteristics, such as gender, age, medical history, exercise program type, number and type of movements, intensity and duration of training, and other program-specific factors. It is important to note that respiratory function is influenced by multiple factors, including the nervous system, neuromuscular coordination, respiratory muscle strength, and lung size. Enhancing respiratory muscle strength and reducing airway resistance through exercise has been shown to improve lung function. Exercise-induced bronchial dilation reduces airway resistance, thereby improving ventilation. Additionally, exercises involving significant muscle engagement can increase breathing rate and depth, which, in turn, enhances FVC, oxygen uptake, and oxygen distribution [46].
Conclusion
The findings of this study indicate that concurrent exercise programs of varying intensities can lead to improvements in selected respiratory and physiological parameters, demonstrating potential benefits for women with type 2 diabetes. Both low- and high-intensity training programs showed some positive effects on specific indicators, suggesting their utility in managing the condition. Based on these results, healthcare professionals and specialists are encouraged to promote such exercise programs as part of a comprehensive management plan for patients with type 2 diabetes to enhance their chances of recovery. However, the study also revealed that a 12-week exercise intervention did not result in significant changes in certain respiratory and physical parameters. This suggests that longer training durations, combined with careful management of confounding factors, such as nutritional habits, may yield more pronounced and consistent improvements.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Sabzevar University of Medical Sciences, Sabzevar, Iran (Code: IR.MEDSAB.REC.1396.93). Written informed consent was obtained from the participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Amirhossein Haghighi and Malihe Ebrahimi; Methodology: Malihe Ebrahimi and Amirhossein Haghighi; Investigation: Malihe Ebrahimi, Roya Askari, Amirhossein Haghighi, Mitra khademosharie; Data collection: Malihe Ebrahimi , Data analysis: Amirhossein Haghighi and Mitra khademosharie; Writing–review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all their colleagues who helped them in carrying out this research project.
References
Diabetes mellitus is a metabolic disorder that causes a lack of insulin production, a deficiency in its action, or both, resulting in hyperglycemia with impaired carbohydrate, lipid, and protein metabolism. The disease is chronic, serious, and progressive, with multiple damages to various organs. Major and long-term complications of diabetes include cardiovascular disease, diabetic nephropathy, diabetic retinopathy, and damage to other organs, such as the lungs. All of these result from macrovascular and microvascular damage [1].
The association between decreased lung function and diabetes has been described for many years, although the clinical significance of this association is unknown [2, 3]. Potential links between respiratory disorders and diabetes may be attributed to factors, such as elevated body mass index, reduced respiratory efficiency, neuropathy, weakened respiratory muscle strength, and other contributing variables [2]. Additionally, biochemical abnormalities, including inadequate glucose regulation, play a critical role in elevating the risk of pulmonary complications and related health conditions in individuals with diabetes [4, 5].
Pathological studies have shown that the causes of pulmonary dysfunction in patients with diabetes are the major changes in lung tissues, such as changes in the thickness of the alveolar wall, the thickness of the alveolar capillaries, and the lining thickness of the arterioles. Researchers have identified the main causes of these respiratory disorders as hyperglycemia and glycosylation of the chest wall muscles [6]. Some other studies have shown that increased respiratory infections, inflammation, and oxidative stress induced by diabetes reduce respiratory muscle function and limit pulmonary function [7-9]. The Women’s cardiovascular health research center of Britannia showed that insulin resistance and diabetes are associated with reductions in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) [10].
Various studies have suggested that physical activities can be effective in the prevention, control, and treatment of diabetes. Regular moderate exercise is effective in preventing and delaying the onset of diabetes mellitus (DM), increases insulin sensitivity, and improves glucose metabolism [11, 12]. Moreover, exercise training has been considered a therapeutic intervention in the pulmonary rehabilitation program of patients with pulmonary dysfunction [13, 14]. However, the type, severity, and duration of these exercises need further investigation. The American College of Sports Medicine and the American Diabetes Association have confirmed that rehabilitation programs for patients with diabetes should combine aerobic and resistance exercises to maximize the beneficial effects of both types of exercise [15].
Combining aerobic exercise with resistance training offers greater benefits than either modality alone in reducing inflammatory markers, regulating blood sugar and insulin function, and improving cardiovascular disease risk factors [16-19]. Despite extensive research on cardiovascular complications and diabetic nephropathy, retinopathy, and neuropathy, reduced pulmonary function in diabetic patients has received comparatively less attention. Additionally, studies investigating the effects of concurrent exercises on pulmonary function are relatively scarce. For instance, Tunkamnerdthai et al. demonstrated that an 8-week push-up exercise program (three sessions per week) improved FEV1, FVC, and maximal voluntary ventilation (MVV) while reducing HbA1c levels, although the FEV1/FVC ratio remained unchanged [20]. Similarly, Osho et al. examined the effects of a 12-week aerobic-strength training program (with and without weights) on the pulmonary function of 60 patients with type 2 diabetes aged 40–75 years. Their findings revealed significant improvements in VO2 max, FEV1, and HbA1c levels, with FVC and FEV1 indices showing greater enhancement in concurrent training with weights compared to training without weights or the control group [21].
Kim et al. stated that exercise training intensity is one of the factors that affect the strength and resistance of respiratory muscles and pulmonary function [22]. Concurrent exercise programs can enhance respiratory muscle strength and endurance, combining aerobic and resistance training components. Aerobic exercises, such as cycling or walking improve oxygen exchange efficiency and lung capacity, while resistance training strengthens the muscles involved in breathing—beneficial for individuals with type 2 diabetes who may experience respiratory muscle weakness due to the disease or a sedentary lifestyle [23]. Additionally, regular exercise has been shown to improve glycemic control, reflected in lower HbA1c levels. Concurrent exercise programs are particularly effective as they combine the immediate blood sugar-lowering effects of aerobic exercise with the longer-term benefits of resistance training on insulin sensitivity [24]. However, the optimal intensity, frequency, and duration of such programs for individuals with type 2 diabetes remain under investigation. Tailoring these programs to an individual’s fitness level, comorbidities, and medication regimen is critical for safety and effectiveness. Further research is warranted to fully elucidate the effects of concurrent exercise programs on respiratory muscle function, body composition, glycemic control, and overall physical condition in this population. Such studies should also address unique considerations for this group, including hypoglycemia risk, medication interactions, and the influence of diet and other lifestyle factors.
However, the effect of intensity of a concurrent exercise program on respiratory muscles, improvement of body composition, HbA1c, and the physical condition of people with type 2 diabetes is not taken into account and needs further investigation. Therefore, this study was designed to investigate the effect of a concurrent exercise training (aerobic and resistance) program with different intensities (high and low) on the pulmonary function of women with type 2 diabetes.
Materials and Methods
Study sample
The research method was quasi-experimental with pre-test and post-test design. The statistical population included obese women with type 2 diabetes who attended the diabetes community in Sabzevar and had medical records in this medical center. Thirty-four individuals with a history of at least five years of diabetes were selected through availability sampling according to the inclusion criteria. The inclusion criteria were women aged 45-60 years (all subjects were evaluated by a specialist and confirmed to be menopausal); a body mass index (BMI) of 27 to 34 kg/m²; no other diseases (such as autoimmune disorders, respiratory diseases, liver disease, cardiac ischemia, kidney disease, chronic inflammatory diseases, thyroid diseases, stomach ulcers, and infection), a diagnosis of type 2 diabetes; fasting blood glucose levels of less than 180 mg/dl; a 2-hour glucose level of less than 250 mg/dl; no insulin injections; non-smokers; no participation in regular sports programs during the past six months; and the ability to attend the study for three months. Individuals who did not meet these conditions were excluded from the study. Subsequently, ten participants refused to continue their participation for personal reasons and due to disease progression. The remaining 24 subjects were randomly assigned to three groups: low-intensity exercise (8 individuals), high-intensity exercise (8 individuals), and a control group (8 individuals). Written informed consent was obtained from all participants.
Training program
The study began at the sports physiology laboratory at Hakim Sabzevari University for anthropometric and physiological measurements on December 5, 2021. Height, weight, body fat percentage, and waist-hip ratio were measured one week before the beginning of the training program. The following two to three sessions were held in the practice hall to familiarize participants with the training conditions. The training intervention consisted of 12 weeks of concurrent exercise, combining resistance and aerobic activities, performed at two intensity levels: High intensity (resistance at 75–85% of 1-RM and aerobic at 70–85% of maximum heart rate) and low intensity (resistance at 50–75% of 1-RM and aerobic at 50–70% of maximum heart rate). Participants completed three nonconsecutive sessions per week, with each session lasting between 55 and 70 minutes.
The training program of high-intensity concurrent exercises group
The program consisted of 10 minutes of warm-up (jogging, hand and foot movements combined with stretching), followed by 30 minutes of strength training and 10 minutes of cool-down. The stations included bench presses, dumbbell bicep curls, triceps pushdowns, seated leg extensions, leg curls, butterfly exercises, and underhand cable pull-downs. The exercises were performed in accordance with Table 1. The rest interval between each station was set at 120 seconds. After a 15-20 minute rest, 20 minutes of aerobic running training was conducted, featuring 10-12 repetitions for 1 minute at 70-85% of the maximum heart rate, with 30 seconds of active rest between sets at 30-40% of the maximum heart rate (Table 1).

The training program of low-intensity concurrent exercises group
This program included 10 minutes of warm-up, 30 minutes of strength training exercises, and 10 minutes of cool-down. The rest interval between stations was considered also set at 120 seconds. After 20 minutes, aerobic running training at low intensity was performed according to Table 2.



Measurements
Blood samples were collected at two stages: Before and 24 hours after the last session, following a 10-12 hour fasting period. Glycosylated hemoglobin (HbA1c) was measured through an enzymatic colorimetric method with the Nicoard kit (made in Norway, catalog number 1042184), which has a coefficient of variation (CV) of less than 5%. To calculate the body fat percentage, a baseline caliper was used to measure subcutaneous fat in three areas: The thigh, upper pelvic area, and arm triceps, along with the Jackson and Pollock formulas [25]. Maximum oxygen consumption was measured using the Rockport 1-mile (1609 m) Walk Test [21, 26]. Indices of respiratory and pulmonary function were measured with a spirometer (model: CHESTGRAPH HI-701, France). The upper body strength was calculated using the bench press movement and the Brzycki Equation 1
1. 1RM=Weight÷(1.0278–(0.0278×number of repetitions)
Provided that the number of repetitions fell between six and ten repetitions. Upper body muscular strength was computed by the press movement and maximum repetitions to fatigue with 60% of 1RM [27, 28].
To measure the specific strength of the respiratory muscles, a spirometer was used [29]. After a full exhale, the subject was asked to place the mouthpiece in their mouth and breathe in sharply. As the subject inhaled, the piston moved upward, and the highest number displayed was considered the specific strength of the respiratory muscles. This test was repeated three times and the maximum value was calculated. At the same time, in order to monitor the participant’s diet, a 24-hour dietary recall questionnaire, and the three-day dietary records from the beginning and end of the study were used. Food data were analyzed using Nutrition software, version 4.
Statistical methods
Data were described as Mean±SD. The normality of the samples’ distribution was assessed using the Shapiro-Wilk test with Lilliefors correction for small samples, demonstrating a normal distribution for all samples. Treatment effects were analyzed using a two-way ANOVA (IBM SPSS software, version 21, Armonk, VA, USA). A significant interaction effect (group×time) indicated a treatment-dependent difference in the development from pre-test to post-test among the group conditions. Significance was accepted for P≤0.05.
Results
This study showed that 12 weeks of concurrent training at different intensities had significant therapeutic effects on some pulmonary variables, HbA1c values, and body fat percentage in women with type 2 diabetes. Table 3 shows the characteristics of the patients. Table 4 provides an overview of all physiological and functional variables, while Table 5 presents an overview of all respiratory variables.
.PNG)

For several respiratory and physiological parameters, a significant interaction effect (group×time: P<0.05) was found, demonstrating a considerable influence of the respective treatments on the development from the pre-test to the post-test: FEF25, FEF50, FEF2575, FEV1/FVC, HbA1c, and fat percentage (Tables 4 and 5).
There were no significant group effects for other respiratory parameters (P>0.05). For some respiratory, physiological, and functional parameters, significant time effects were observed (P<0.05), indicating a significant change between the pre-test and post-test that was independent of the type of treatment: FVC, FEV, peak expiratory flow (PEF), FEF75, MVV, VO2max, upper body power, and upper body endurance (Tables 4 and 5).
Discussion
This study demonstrated that a 12-week concurrent training program with varying intensities had significant therapeutic effects on certain pulmonary variables, HbA1c levels, and body fat percentage in women with type 2 diabetes. These findings align with those of Silva-Reis et al. who reported that concurrent training enhances lung mechanics and reduces lung inflammation in overweight and obese women [30]. Similarly, Silva-Reis et al. found that concurrent exercise improved lung function, mechanics, and pulmonary immune responses in overweight and stage 1 obese women by elevating levels of the anti-fibrotic protein Klotho while reducing fibrotic IGF-1 [31]. These improvements were attributed to IGF-1, which is recognized as a primary growth factor overproduced in conditions, such as asthma and obesity. In asthma, IGF-1 plays a role in modulating airway inflammation, smooth muscle hyperresponsiveness, and hypertrophy, ultimately affecting lung mechanics [32]. Tunkamnerdthai et al. observed that an eight-week arm swing exercise program (three sessions per week) led to improvements in FEV1, FVC, and MVV while lowering HbA1c levels; however, the FEV1/FVC ratio remained unchanged in their study [20].
Osho et al. also documented significant enhancements in VO2max, FVC, FEV1, and HbA1c levels among 60 individuals with type 2 diabetes aged between 40 and 75 years [26]. Additionally, Chen et al. reported a notable increase in PEF following an eight-week kettlebell training program, indicating improved pulmonary function after exercise [33]. These findings suggest that the respiratory muscles of the participants in this study were effectively trained, resulting in significant improvements in PEF. While prior studies on resistance training in older adults have primarily examined its benefits for individuals with lung injuries or other specific conditions, fewer studies have focused on markers of lung function in this population [34].
Contrary to our findings, Jones and Nzekwu [35] reported no significant changes in lung volume. The results of our study can be explained by the acute enhancements in glucose metabolism that occur immediately after exercise, with these effects lasting for up to 48 hours, as noted by Davis et al. [36].
Poor glucose regulation is linked to microvascular complications and pulmonary respiratory distress [37]. Dyspnea is the leading cause of death in type 2 diabetes after adjusting for other known risk factors [38]. The reduction in mean HbA1c levels observed in this study, which aligns with findings from other research [37, 38] but contrasts with those reported by Sigal et al. [39], may contribute to significant improvements in FEV1/FVC values.
FVC is influenced by factors, such as age, physical activity level, body composition and health, respiratory muscle strength, and lung elasticity [40]. Additionally, the extent of lung volume reduction and airflow limitation correlates with glucose levels and body fat [41]. Hence, the increase in FVC observed in this study following concurrent training may be attributed to enhanced respiratory muscle strength and power, reduced body fat, and lower fasting blood glucose in patients with diabetes. However, the 12-week concurrent training program did not lead to a significant increase in FEV1 among women with type 2 diabetes in either training group. This outcome contrasts with findings from studies conducted by Osho et al. and Beckerman et al. [42]. FEV1, a specific measure of respiratory function, is affected by various factors. A decline in FEV1 indicates reduced lung capacity, airway obstruction, decreased lung reversibility, or, less commonly, insufficient respiratory muscle development. Thus, improving respiratory muscle strength can also enhance FEV1 [40]. As FEV1 serves as an indicator of respiratory muscle strength, it appears that exercises targeting the enhancement of respiratory muscle strength may contribute to increased FEV1 levels [43].
Many studies have reported reduced pulmonary function in diabetic patients, largely due to diminished strength and power of the respiratory muscles. In the present study, while upper and lower body muscle strength and power increased in both the low- and high-intensity training groups, these changes were not statistically significant. Although the strengthened muscles in this study primarily served as supporting muscles for pulmonary function rather than the primary muscles involved in respiration, their long-term strengthening appeared to contribute to improvements in respiratory system function. Additionally, aerobic training may enhance the diaphragm, intercostal, and abdominal muscles while reducing resistance in the respiratory tract. Notably, the specific strength of respiratory muscles, as measured by spirometry, improved in the low-intensity concurrent training group. This underscores the combined role of aerobic and resistance exercises in promoting pulmonary function. Prior research has also shown a positive and significant correlation between cardiovascular fitness (VO2max) and pulmonary function indices, such as FEV1 and FVC [44, 45].
The respiratory and cardiovascular systems collaborate closely to deliver oxygen to cells and regulate the internal environment of the body during both rest and activity. This partnership ensures a balance between ventilation and cardiac function in the gas exchange process that links skeletal muscles to atmospheric air. Any inefficiency in these systems can impair overall body function. When the ventilation-to-blood flow ratio or the ventilation-to-oxygen absorption and removal ratio declines relative to ventilation volume, respiratory muscles consume more energy, increasing the likelihood of premature fatigue [45]. Although the current study observed improvements in maximum oxygen consumption (VO2max) in both training groups, these changes were not statistically significant. Nevertheless, this factor may partially explain the observed enhancements in pulmonary function. This study demonstrated improvements across several key indicators, including respiratory muscle strength and power, body fat percentage, body weight, physical fitness, aerobic capacity, blood glucose levels, and HbA1c among the participants. The variability in results across studies may stem from differences in participant characteristics, such as gender, age, medical history, exercise program type, number and type of movements, intensity and duration of training, and other program-specific factors. It is important to note that respiratory function is influenced by multiple factors, including the nervous system, neuromuscular coordination, respiratory muscle strength, and lung size. Enhancing respiratory muscle strength and reducing airway resistance through exercise has been shown to improve lung function. Exercise-induced bronchial dilation reduces airway resistance, thereby improving ventilation. Additionally, exercises involving significant muscle engagement can increase breathing rate and depth, which, in turn, enhances FVC, oxygen uptake, and oxygen distribution [46].
Conclusion
The findings of this study indicate that concurrent exercise programs of varying intensities can lead to improvements in selected respiratory and physiological parameters, demonstrating potential benefits for women with type 2 diabetes. Both low- and high-intensity training programs showed some positive effects on specific indicators, suggesting their utility in managing the condition. Based on these results, healthcare professionals and specialists are encouraged to promote such exercise programs as part of a comprehensive management plan for patients with type 2 diabetes to enhance their chances of recovery. However, the study also revealed that a 12-week exercise intervention did not result in significant changes in certain respiratory and physical parameters. This suggests that longer training durations, combined with careful management of confounding factors, such as nutritional habits, may yield more pronounced and consistent improvements.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Sabzevar University of Medical Sciences, Sabzevar, Iran (Code: IR.MEDSAB.REC.1396.93). Written informed consent was obtained from the participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Amirhossein Haghighi and Malihe Ebrahimi; Methodology: Malihe Ebrahimi and Amirhossein Haghighi; Investigation: Malihe Ebrahimi, Roya Askari, Amirhossein Haghighi, Mitra khademosharie; Data collection: Malihe Ebrahimi , Data analysis: Amirhossein Haghighi and Mitra khademosharie; Writing–review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all their colleagues who helped them in carrying out this research project.
References
- Kopf S, Kumar V, Kender Z, Han Z, Fleming T, Herzig S, et al. Diabetic pneumopathy-A new diabetes-associated complication: Mechanisms, consequences and treatment considerations. Frontiers in Endocrinology. 2021; 12:765201. [DOI:10.3389/fendo.2021.765201] [PMID]
- Ozoh OB, Okubadejo NU, Chukwu CC, Bandele EO. Eligibility of Nigerians with type 2 diabetes mellitus for inhaled insulin. Nigerian Quarterly Journal of Hospital Medicine. 2010; 20(2):77-80. [DOI:10.4314/nqjhm.v20i2.58037] [PMID]
- Ramalho SHR, Shah AM. Lung function and cardiovascular disease: A link. Trends in Cardiovascular Medicine. 2021; 31(2):93-8. [DOI:10.1016/j.tcm.2019.12.009] [PMID]
- Kaminski DM, Schaan BD, da Silva AM, Soares PP, Plentz RD, Dall'Ago P. Inspiratory muscle weakness is associated with autonomic cardiovascular dysfunction in patients with type 2 diabetes mellitus. Clinical Autonomic Research. 2011; 21(1):29-35. [DOI:10.1007/s10286-010-0087-1] [PMID]
- Wang D, Ma Y, Tong X, Zhang Y, Fan H. Diabetes mellitus contributes to idiopathic pulmonary fibrosis: A review from clinical appearance to possible pathogenesis. Frontiers in Public Health. 2020; 8:196. [DOI:10.3389/fpubh.2020.00196] [PMID]
- Brock JM, Billeter A, Müller-Stich BP, Herth F. Obesity and the lung: What we know today. Respiration. 2020; 99(10):856-66. [DOI:10.1159/000509735] [PMID]
- Klein OL, Krishnan JA, Glick S, Smith LJ. Systematic review of the association between lung function and type 2 diabetes mellitus. Diabetic Medicine. 2010; 27(9):977-87. [DOI:10.1111/j.1464-5491.2010.03073.x] [PMID]
- El-Habashy MM, Agha MA, El-Basuni HA. Impact of diabetes mellitus and its control on pulmonary functions and cardiopulmonary exercise tests. Egyptian Journal of Chest Diseases and Tuberculosis. 2014; 63(2):471-6. [DOI:10.1016/j.ejcdt.2013.11.003]
- Shah SH, Sonawane P, Nahar P, Vaidya S, Salvi S. Pulmonary function tests in type 2 diabetes mellitus and their association with glycemic control and duration of the disease. Lung India. 2013; 30(2):108-12. [DOI:10.4103/0970-2113.110417] [PMID]
- Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: Findings from the British Women's Heart and Health Study. Diabetologia. 2004; 47(2):195-203. [DOI:10.1007/s00125-003-1310-6] [PMID]
- Acosta-Manzano P, Rodriguez-Ayllon M, Acosta FM, Niederseer D, Niebauer J. Beyond general resistance training. Hypertrophy versus muscular endurance training as therapeutic interventions in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Obesity Reviews. 2020; 21(6):e13007. [DOI:10.1111/obr.13007] [PMID]Amanat S, Ghahri S, Dianatinasab A, Fararouei M, Dianatinasab M. Exercise and type 2 diabetes. Advances in Experimental Medicine and Biology. 2020; 1228:91-105. [DOI:10.1007/978-981-15-1792-1_6] [PMID]
- Park J, Han D. Effects of high intensity aerobic exercise on treadmill on maximum-expiratory lung capacity of elderly women. Journal of Physical Therapy Science. 2017; 29(8):1454-7. [DOI:10.1589/jpts.29.1454]
- İşleyen G, Dağlıoğlu Ö. The effect of aerobic exercise on pulmonary function and aerobic capacity in sedentary men. International Journal of Sport Exercise and Training Sciences-IJSETS. 2020; 6(3):80-7. [DOI:10.18826/useeabd.784339]
- Kobayashi Y, Long J, Dan S, Johannsen NM, Talamoa R, Raghuram S, et al. Strength training is more effective than aerobic exercise for improving glycaemic control and body composition in people with normal-weight type 2 diabetes: A randomised controlled trial. Diabetologia. 2023; 66(10):1897-907. [DOI:10.1007/s00125-023-05958-9] [PMID]
- Tan S, Li W, Wang J. Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. Journal of Sports Science & Medicine. 2012; 11(3):495. [PMID]
- Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Annals of Internal Medicine. 2007; 147(6):357-69. [DOI:10.7326/0003-4819-147-6-200709180-00005] [PMID]
- Brown EC, Franklin BA, Regensteiner JG, Stewart KJ. Effects of single bout resistance exercise on glucose levels, insulin action, and cardiovascular risk in type 2 diabetes: A narrative review. Journal of Diabetes and Its Complications. 2020; 34(8):107610. [DOI:10.1016/j.jdiacomp.2020.107610] [PMID]
- Brinkmann C, Weh-Gray O, Bloch W, Brixius K, Predel HG, Kreutz T. Effects of a combined endurance/strength training program on circulating irisin levels in overweight/obese men and women with type 2 diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2022; 130(1):37-42. [DOI:10.1055/a-1284-5428] [PMID]
- Tunkamnerdthai O, Auvichayapat P, Donsom M, Leelayuwat N. Improvement of pulmonary function with arm swing exercise in patients with type 2 diabetes. Journal of Physical Therapy Science. 2015; 27(3):649-54. [DOI:10.1589/jpts.27.649] [PMID]
- Osho OA, Akinbo S, Osinubi A, Olawale O. Effect of weight bearing and non-weight bearing aerobics combined with resistance exercises on the cardiopulmonary functions of Nigerians with type 2 diabetes mellitus. Journal of Diabetes & Metabolic Disorders. 2011; 10:2. [Link]
- Kim AR, Shin WS. Effects of high-intensity intermittent training and moderate-intensity training on cardiopulmonary capacity in Canoe and Kayak Paddlers during 8 weeks. Journal of The Korean Society of Physical Medicine. 2014; 9(3):307-14. [DOI:10.13066/kspm.2014.9.3.307]
- Xiong T, Bai X, Wei X, Wang L, Li F, Shi H, et al. Exercise rehabilitation and chronic respiratory diseases: Effects, mechanisms, and therapeutic benefits. International Journal of Chronic Obstructive Pulmonary Disease. 2023; 18:1251-66. [DOI:10.2147/COPD.S408325] [PMID]
- Bassi D, Mendes RG, Arakelian VM, Caruso FC, Cabiddu R, Júnior JC, et al. Potential effects on cardiorespiratory and metabolic status after a concurrent strength and endurance training program in diabetes patients-A randomized controlled trial. Sports Medicine-Open. 2016; 2:31. [DOI:10.1186/s40798-016-0052-1] [PMID]
- Jackson AS, Pollock ML. Practical assessment of body composition. The Physician and Sportsmedicine. 1985; 13(5):76-90. [DOI:10.1080/00913847.1985.11708728] [PMID]
- Osho O, Akinbo S, Osinubi A, Olawale O. Effect of progressive aerobic and resistance exercises on the pulmonary functions of individuals with type 2 diabetes in Nigeria. International Journal of Endocrinology and Metabolism. 10(1):411-7. [DOI:10.5812/ijem.3333]
- Mayhew JL, Johnson BD, Lamonte MJ, Lauber D, Kemmler W. Accuracy of prediction equations for determining one repetition maximum bench press in women before and after resistance training. Journal of Strength and Conditioning Research. 2008; 22(5):1570-7. [DOI:10.1519/JSC.0b013e31817b02ad] [PMID]
- Mayhew JL, Prinster JL, Ware JS, Zimmer DL, Arabas JR, Bemben MG. Muscular endurance repetitions to predict bench press strength in men of different training levels. The Journal of Sports Medicine and Physical Fitness. 1995; 35(2):108-13. [PMID]
- Turner LA, Mickleborough TD, McConnell AK, Stager JM, Tecklenburg-Lund S, Lindley MR. Effect of inspiratory muscle training on exercise tolerance in asthmatic individuals. Medicine and Science in Sports and Exercise. 2011; 43(11):2031-8. [DOI:10.1249/MSS.0b013e31821f4090] [PMID]
- Silva-Reis A, Brandao-Rangel MA, Moraes-Ferreira R, Gonçalves-Alves TG, Souza-Palmeira VH, Aquino-Santos HC, et al. Effects of combined physical training on lung function and mechanics and on pulmonary immune response in overweight and obese women. European Respiratory Journal. 58(suppl 65):PA3913. [DOI:10.1183/13993003.congress-2021.PA3913]
- Silva-Reis A, Rodrigues Brandao-Rangel MA, Moraes-Ferreira R, Gonçalves-Alves TG, Souza-Palmeira VH, Aquino-Santos HC, et al. Combined resistance and aerobic training improves lung function and mechanics and fibrotic biomarkers in overweight and obese women. Frontiers in Physiology. 2022; 13:946402. [DOI:10.3389/fphys.2022.946402] [PMID]
- Han YY, Yan Q, Chen W, Forno E, Celedón JC. Serum insulin-like growth factor-1, asthma, and lung function among British adults. Annals of Allergy, Asthma & Immunology. 2021; 126(3):284-91.e2. [DOI:10.1016/j.anai.2020.12.005] [PMID]
- Chen HT, Wu HJ, Chen YJ, Ho SY, Chung YC. Effects of 8-week kettlebell training on body composition, muscle strength, pulmonary function, and chronic low-grade inflammation in elderly women with sarcopenia. Experimental Gerontology. 2018; 112:112-8. [DOI:10.1016/j.exger.2018.09.015] [PMID]
- Grisbrook TL, Wallman KE, Elliott CM, Wood FM, Edgar DW, Reid SL. The effect of exercise training on pulmonary function and aerobic capacity in adults with burn. Burns. 2012; 38(4):607-13. [DOI:10.1016/j.burns.2012.03.007]
- Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006; 130(3):827-33. [DOI:10.1378/chest.130.4.941] [PMID]
- Davis WA, Knuiman M, Kendall P, Grange V, Davis TM; Fremantle Diabetes Study. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004; 27(3):752-7. [DOI:10.2337/diacare.27.3.752] [PMID]
- Davis TM, Knuiman M, Kendall P, Vu H, Davis WA. Reduced pulmonary function and its associations in type 2 diabetes: The fremantle diabetes study. Diabetes Research and Clinical Practice. 2000; 50(2):153-9. [DOI:10.1016/S0168-8227(00)81978-6] [PMID]
- McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: An analysis of data from the third national health and nutrition examination survey. American Journal of Epidemiology. 2005; 161(6):546-56. [DOI:10.1093/aje/kwi076] [PMID]
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004; 27(10):2518-39. [DOI:10.2337/diacare.27.10.2518] [PMID]
- Womack CJ, Harris DL, Katzel LI, Hagberg JM, Bleecker ER, Goldberg AP. Weight loss, not aerobic exercise, improves pulmonary function in older obese men. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2000; 55(8):M453-7. [DOI:10.1093/gerona/55.8.M453] [PMID]
- Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity. 2009; 17(3):578-84. [DOI:10.1038/oby.2008.584] [PMID]
- Beckerman M, Magadle R, Weiner M, Weiner P. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest. 2005; 128(5):3177-82. [DOI:10.1378/chest.128.5.3177] [PMID]
- Figueiredo RIN, Azambuja AM, Cureau FV, Sbruzzi G. Inspiratory Muscle Training in COPD. Respiratory Care. 2020; 65(8):1189-201. [DOI:10.4187/respcare.07098] [PMID]
- Rabiee MA, Ghanbarzadeh M, Habibi A, Marashiyan H. [Relationship between body composition and cardiorespiratory fitness with pulmonary function in light weight and heavy weight professional Greco-Roman wrestlers (Persian)]. Shomal Journal of Management and Physiology in Sport. 2014; 1(1):13-20. [Link]
- Haines DA, Wilby K. Relationship between lung function and physical fitness in 9 to 15 year old Australian children, Australian Journal of Science and Medicine In Sport. 1993; 25(1993):35-9. [Link]
- Fatima SS, Rehman R, Saifullah, Khan Y. Physical activity and its effect on forced expiratory volume. The Journal of the Pakistan Medical Association. 2013; 63(3):310-2. [PMI
Type of Study: Research |
Subject:
Sport injury and corrective exercises
Received: 2024/07/23 | Accepted: 2025/01/4 | Published: 2025/07/13
Received: 2024/07/23 | Accepted: 2025/01/4 | Published: 2025/07/13
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



