Thu, Feb 19, 2026
Volume 16, Issue 1 (Winter 2026)
PTJ 2026, 16(1): 49-62 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mousavian F, Shamsipour Dehkordi P, khalaji M. Comparison of Central and Peripheral Fatigue Effects on Golf Swing Variability and Smoothness. PTJ 2026; 16 (1) :49-62
URL: http://ptj.uswr.ac.ir/article-1-651-en.html
URL: http://ptj.uswr.ac.ir/article-1-651-en.html
1- Department of Motor Behavior, Faculty of Sport Sciences, Alzahra University, Tehran, Iran.
2- Department of Sports Coaching, Faculty of Sports and Health Sciences, University of Tehran, Tehran, Iran.
2- Department of Sports Coaching, Faculty of Sports and Health Sciences, University of Tehran, Tehran, Iran.
Full-Text [PDF 815 kb]
(896 Downloads)
| Abstract (HTML) (1384 Views)
Full-Text: (249 Views)
Introduction
Motor activity constitutes a fundamental aspect of human movement, shaped by individual capacity, experience, and environmental adaptations. In precision-based sports, such as golf, where coordination and biomechanical efficiency are paramount, fatigue represents a critical factor influencing performance. Motor coordination relies on neuromuscular control to facilitate smooth and efficient movements [1]. However, fatigue disrupts this process, inducing variations in movement patterns, increasing error rates, and heightening the risk of injury [2].
Arises from prolonged physical exertion, leading to a decline in the neuromuscular system’s capacity for force production and inter-muscular synchronization [3]. According to Tornero-Aguilera et al. central and peripheral fatigue have been broadly explained in exercise physiology literature [4]. Furthermore, Izadi et al. reported that both central and peripheral fatigue negatively affect movement coordination and accuracy in handball players [5]. Similarly, Ortiz et al. emphasize that fatigue-induced alterations in motor coordination negatively affect joint stability, particularly in sports requiring repetitive movement patterns [6]. Given these implications, it is hypothesized that muscular fatigue detrimentally affects motor coordination in golf, increasing variability in movement execution.
Conversely, central fatigue results from sustained cognitive exertion, impairing central nervous system activity and potentially disrupting motor control and proprioception [7, 8]. Tornero-Aguilera et al. suggest that central fatigue disrupts cortical processing, leading to prolonged reaction times and diminished movement accuracy [4]. Studies in other sports, such as soccer, show that central fatigue negatively affects passing decision-making and cognitive-motor integration [9]. Similarly, Smith et al. demonstrate that central fatigue significantly compromises decision-making and motor execution in precision-dependent sports, such as golf [10]. Neuroscientific research indicates that mental exhaustion alters neuromuscular responses, disrupts proprioception, and hinders motor planning due to its influence on the frontal cortex, ultimately impairing performance [11].
Understanding the effects of fatigue is especially critical in sports requiring both physical endurance and cognitive acuity. Golf presents a unique challenge, demanding precise motor execution, sustained concentration, and biomechanical efficiency [12-14]. Unlike more physically intensive sports, where muscular endurance is paramount, golf relies on maintaining stable and controlled movement patterns over extended periods [13]. Competitive golf requires substantial cognitive engagement, motor coordination, and endurance [13]. The impact of fatigue on golf performance extends beyond muscular exhaustion, involving complex interactions between neuromuscular and cognitive functions.
Empirical evidence indicates that fatigue significantly impacts the biomechanics of the golf swing [12]. Hakukawa et al. found that fatigue-induced changes in trunk and lower-limb kinematics directly affect swing mechanics and shot accuracy [14]. Research on movement analysis techniques highlights the reliability of motion tracking systems for assessing motor performance under fatigue [15]. Additionally, mental fatigue has been shown to negatively affect functional performance tests, impairing motor planning, decision-making, and execution [16]. Similarly, Gebel et al. reported that postural control declines under fatigue, leading to increased movement variability and reduced shot precision in golfers [17].
Central fatigue also impairs performance, with golfers exhibiting slower reaction times, decreased movement accuracy, and greater kinematic variability [13]. Studies indicate that central fatigue diminishes movement automaticity, forcing athletes to rely more on conscious control, which paradoxically increases variability and reduces performance consistency [8]. Athletes’ perceived exertion, measured via the Borg RPE scale, correlates with both physical and mental fatigue, affecting performance outcomes [18]. Heart rate variability has also been shown to reflect fatigue levels and predict decrements in motor control [19]. Central and peripheral fatigue together impair coordination and timing, further compromising precision-dependent tasks [20]. Neuromuscular rehabilitation research highlights that fatigued individuals exhibit altered motor recruitment patterns and delayed response times [21]. Mental fatigue specifically impairs endurance and cognitive function, reducing the ability to maintain optimal performance under prolonged cognitive load [22]. Cognitive exhaustion has been demonstrated to decrease cognitive flexibility, thereby affecting decision-making and motor planning in sports contexts [23]. Collectively, these effects result in suboptimal motor planning and execution [24].
Addressing this gap, this study aimed to optimize training protocols, fatigue management strategies, and injury prevention measures in golf. Central fatigue is hypothesized to have a more pronounced effect due to its direct influence on cognitive processing and neuromuscular responses [22]. Understanding the distinct effects of central and peripheral fatigue on golf performance could offer valuable insights for enhancing endurance strategies and mitigating performance decline in athletes, ultimately improving both athletic performance and injury prevention [14].
Given these considerations, the present study systematically compared the effects of central and peripheral fatigue on motor coordination in the golf swing to determine which type of fatigue more significantly impacts performance. Notably, fatigue—whether muscular or mental—disrupts motor regulation by altering control strategies, increasing movement inconsistencies, and reducing accuracy. Given the established influence of fatigue on movement variability and smoothness, the study hypothesized that:
1) Both types of fatigue will negatively affect movement variability and smoothness; 2) central fatigue will induce greater variability in motor coordination patterns than peripheral fatigue, as it more substantially impairs central motor control mechanisms [10].
Materials and Methods
This study employed an applied, semi-experimental research design, utilizing pre-test and post-test procedures across three groups: Central fatigue, peripheral fatigue, and a non-intervention group. The statistical population consisted of female students. G*Power software, version 5.3.3 was used to estimate the sample size, with a test power of 0.8, an effect size of 0.8, and an alpha level of 0.05. Participants were selected using criterion-based purposive sampling.
The final sample included 30 young, healthy female sports science students, aged 20–35 years. Eligibility criteria required participants to have no prior experience in golf, be right-handed, have normal or corrected-to-normal vision, be free of underlying health conditions, abstain from regular alcohol or tobacco use, and be in good physical and mental health. During the familiarization session, all testing methods and equipment were explained. To ensure consistency, participants were instructed to get adequate rest (a minimum of eight hours), avoid caffeine, alcohol, heavy exercise, and mentally demanding tasks 24 hours before testing, and consume a nutritious meal approximately 1.5 hours before each session.
Testing sessions were conducted at least 72 hours apart. Individuals were excluded if they had a history of neurological disorders, took medications affecting cortical function, or sustained lower-limb musculoskeletal injuries (e.g. ankle sprains) within the past six months. Written informed consent was obtained after full disclosure of the study’s objectives and protocols.
Procedure
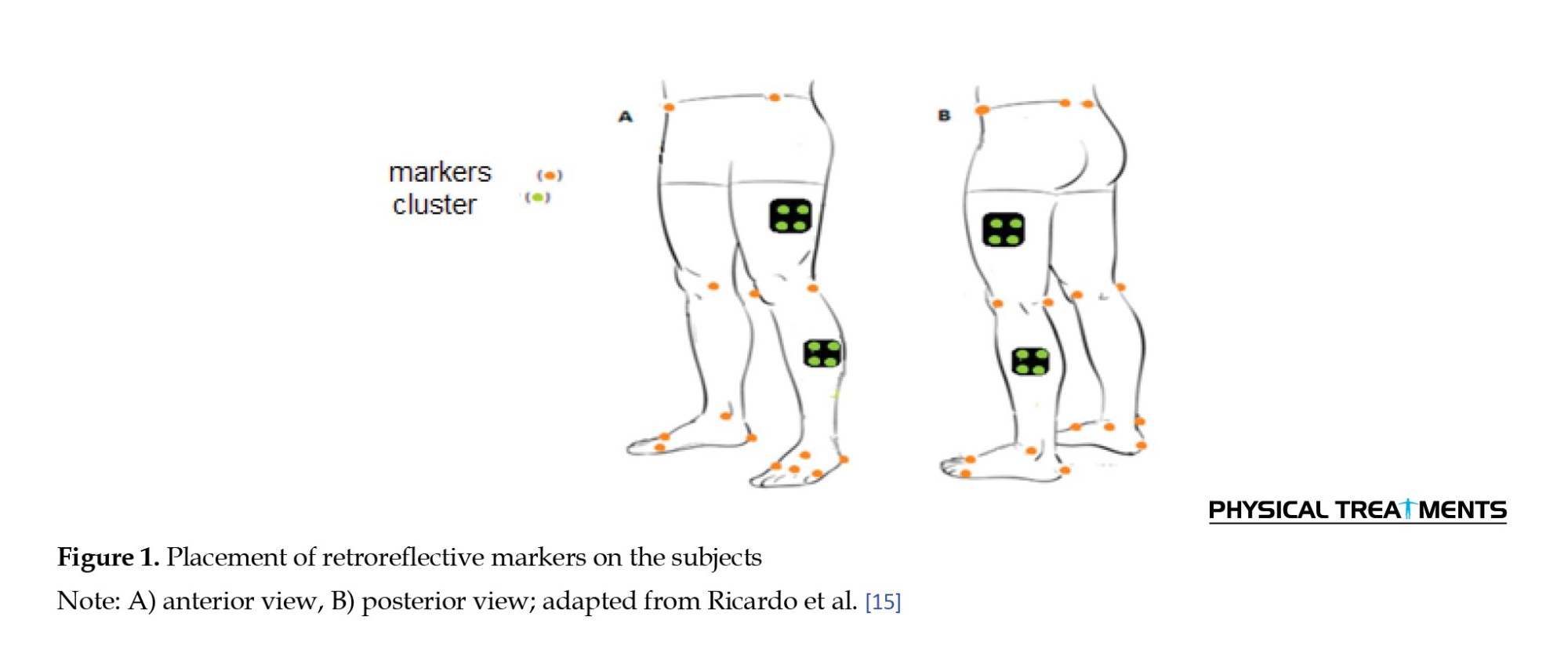
Initially, the subjects hit a 2-meter target three times to warm up and become comfortable with the technique. Each participant was then given ten attempts to hit the target to complete the pre-test. A total of 48 optical markers were attached to the skin, and eight motion analysis cameras with a sampling rate of 240 Hz recorded the subject’s movements during the stroke (Figures 1 and 2). Participants were instructed to stop the ball as close to the target as possible. Twenty-four hours later, the fatigue task was administered first to the peripheral fatigue group. To prevent physical injury, each subject followed a 10-minute protocol involving stretching and dynamic movements, such as jumping and squatting (including sleeping, sitting, standing, and jumping). Subsequently, a modified Borg scale plank exercise was performed for 15 minutes [14].
A score of more than 17 on this scale indicated that the subjects felt fatigued and weak. During the exercises, a heart rate monitor was used to track participant activity and heart rate. The participants’ heart rates increased to 70% of their maximum, and they continued exercising until exhaustion. After completing the fatigue protocol, the subjects took ten golf shots toward the target.
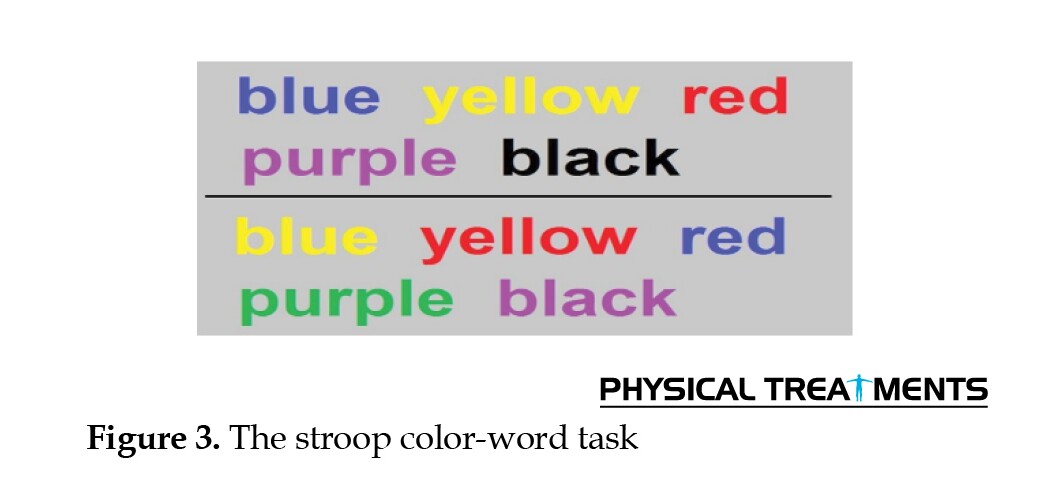
In the central fatigue group, the central fatigue intervention was applied first. Participants in this group first completed the visual analog scale (VAS) to measure their baseline central fatigue levels. They then performed the Stroop task for 45 minutes (Figure 3). After the Stroop task, they completed the VAS again. If participants scored at least 50 points on the VAS, it indicated that they had reached the desired level of central fatigue [10]. In such cases, they continued the Stroop task while completing the VAS every 10 minutes until the intended level of central fatigue was achieved.
In the non-intervention group, participants performed a block of ten golf swings during both the pre-test and retention stages. They rated their perceived exertion using a calibrated Borg scale (ranging from 6 to 20.23) at the start and end of each session. Perceived exertion was defined as the conscious sensation of how hard or strenuous the activity felt [18].
Motion analysis system to record changes in body angles
A motion capture system consisting of eight infrared cameras (AM6110, Bertec, Columbus, OH, USA; frequency: 600 Hz) was used, with data sampled at 250 Hz (Oqus, Qualisys, Sweden). A total of 48 reflective markers were placed on standardized bony landmarks. Marker motion was recorded using Qualisys Track Manager software, version 2.7. Kinematic data and swing speed were calculated using Visual3D (C-Motion, Rockville, MD, USA) [14].
Subjective level of central fatigue
Participants rated their perceived fatigue levels on the VAS, ranging from 0 (not physically/mentally fatigued at all) to 10 (extremely physically/mentally fatigued), to assess subjective levels of physical and mental fatigue (PF/MF) [16]. Subjective measures of central fatigue were taken before and after the fatigue protocols, as previously documented by Verschueren et al. [16]. To confirm central fatigue induction, participants rated their perceived fatigue on the VAS (0–10) before and after the task. An increase in perceived fatigue post-intervention verified central fatigue establishment. This method aligns with prior research indicating that a post-task VAS score increase of at least 3 points reliably indicates central fatigue [16].
All participants in the central fatigue group successfully completed the test. To induce central fatigue, players performed a 30-minute computerized Stroop color-word task, which has been widely used in sports research due to its effectiveness in inducing central fatigue [17]. In this cognitive task, four words (red, blue, green, yellow) appeared individually on a grey-background computer screen. Participants responded by pressing a key corresponding to the word’s color rather than its meaning (e.g. if red appeared in blue, the correct response was the blue key).
To increase difficulty and attention demands, an exception was applied: if the word’s color was red, participants had to press the key matching the word’s meaning (e.g. if green appeared in red, the correct response was the green key).
The task consisted of 50% congruent trials (word and color matching) and 50% incongruent trials. Each word appeared for 1000 ms, followed by a 1000-ms black screen before the next word appeared, resulting in a new stimulus every 2000 ms (900 total stimuli). Incorrect or delayed responses (>1500 ms) triggered a beep to prompt faster or more accurate responses. To enhance motivation, participants were challenged to complete as many correct responses as possible within the 30-minute period while competing for speed and accuracy against others [17].
Rating of perceived exertion
Perceived exertion was recorded using the Borg scale (ranging from 6 to 20) at standardized intervals, both at the start and end of each game [18]. Perceived exertion reflects the subjective cognitive appraisal of physical effort intensity during activity [19].
Peripheral fatigue task
Peripheral fatigue was induced through progressive plank exercises under the researcher’s supervision [14]. The plank is an isometric exercise that promotes muscular fatigue via sustained contraction, resulting in a progressive decline in neuromuscular efficiency—consistent with the study’s objective of examining fatigue’s impact on motor coordination [20]. As a widely used whole-body exercise targeting the trunk, the plank is considered a high-intensity workout.
The forearm plank was performed in accordance with ACSM guidelines: “Body weight supported on forearms and toes, with scapulae protracted and pelvis posteriorly tilted.” To ensure controlled and consistent fatigue induction, participants maintained the plank position until reaching a perceived exertion level of 17 or higher on the modified Borg Scale (indicating “extremely difficult” exertion). Upon reaching this threshold, participants were instructed to stop. Following the plank task, a 30-second rest period was provided before participants performed the golf swing task. This approach standardized peripheral fatigue induction while minimizing the risk of injury and maintaining control over task intensity [14].
Heart rate monitoring
To monitor heart rate and confirm that participants reached approximately 90% of their maximal theoretical heart rate during the fatigue session—equivalent to the average heart rate observed during a badminton match—each participant wore a Polar RS400 running computer [19].
Statistical methods
The Shapiro-Wilk test was used to check the normality of data distribution, and Levene’s test was used to assess the homogeneity of variances. To investigate the main effects of fatigue type (between-group differences) and assessment phase (within-group differences), as well as the interaction effect of fatigue type with assessment phase, a mixed ANOVA with repeated measures (2×3) and Bonferroni’s post hoc test was used. Data analysis was conducted using SPSS software, version 20.
Results
The Mean±SD of wrist jerk are presented in Table 1. In the post-test, the mean wrist jerk in the peripheral fatigue and central fatigue groups was higher than in the control group. A one-way analysis of variance revealed no significant difference in the mean jerk movements between the groups during the pre-test phase (F(2, 27)=0.12, P=0.87).To compare the mean wrist jerk movements, a Mixed ANOVA with repeated measures (2×3) was conducted, followed by a Bonferroni post hoc test (Table 2).
The results of the repeated measures ANOVA (Table 2) revealed a significant main effect of the assessment phase (P=0.001). Examination of the means showed that the mean jerk of movement in the post-test (M=370.76) was higher than in the pre-test (M=353.61). The main effect of group was not statistically significant (P=0.26). However, the interaction effect of assessment phase×group was significant (P=0.001). Pairwise comparisons (see interactive Figure 4) indicated a significant difference in the mean jerk of movement between the control group and both the central fatigue and peripheral fatigue groups (P<0.05).
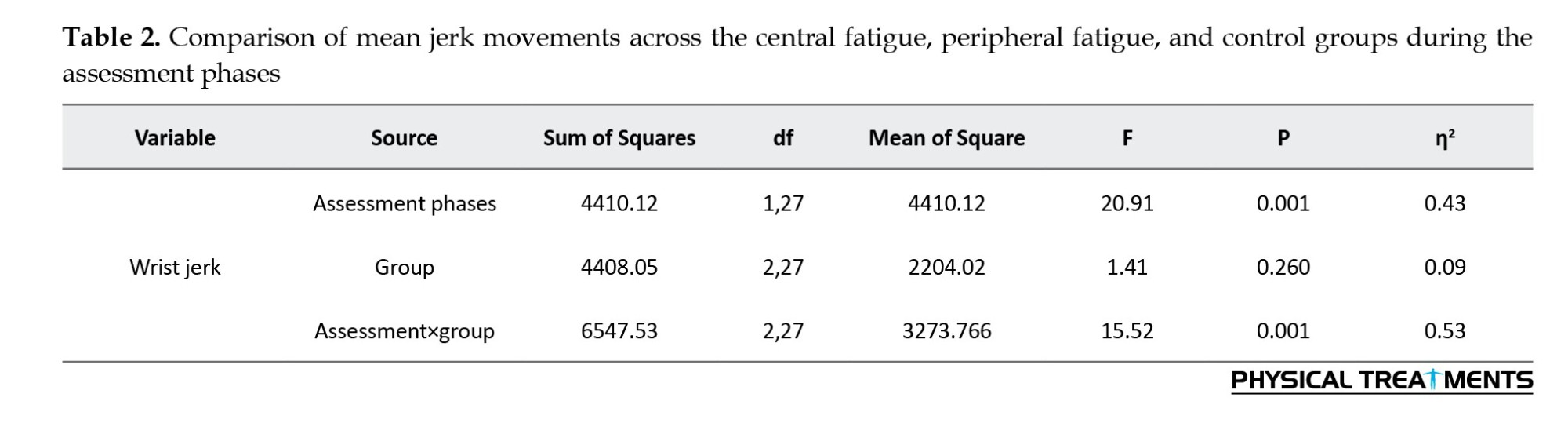
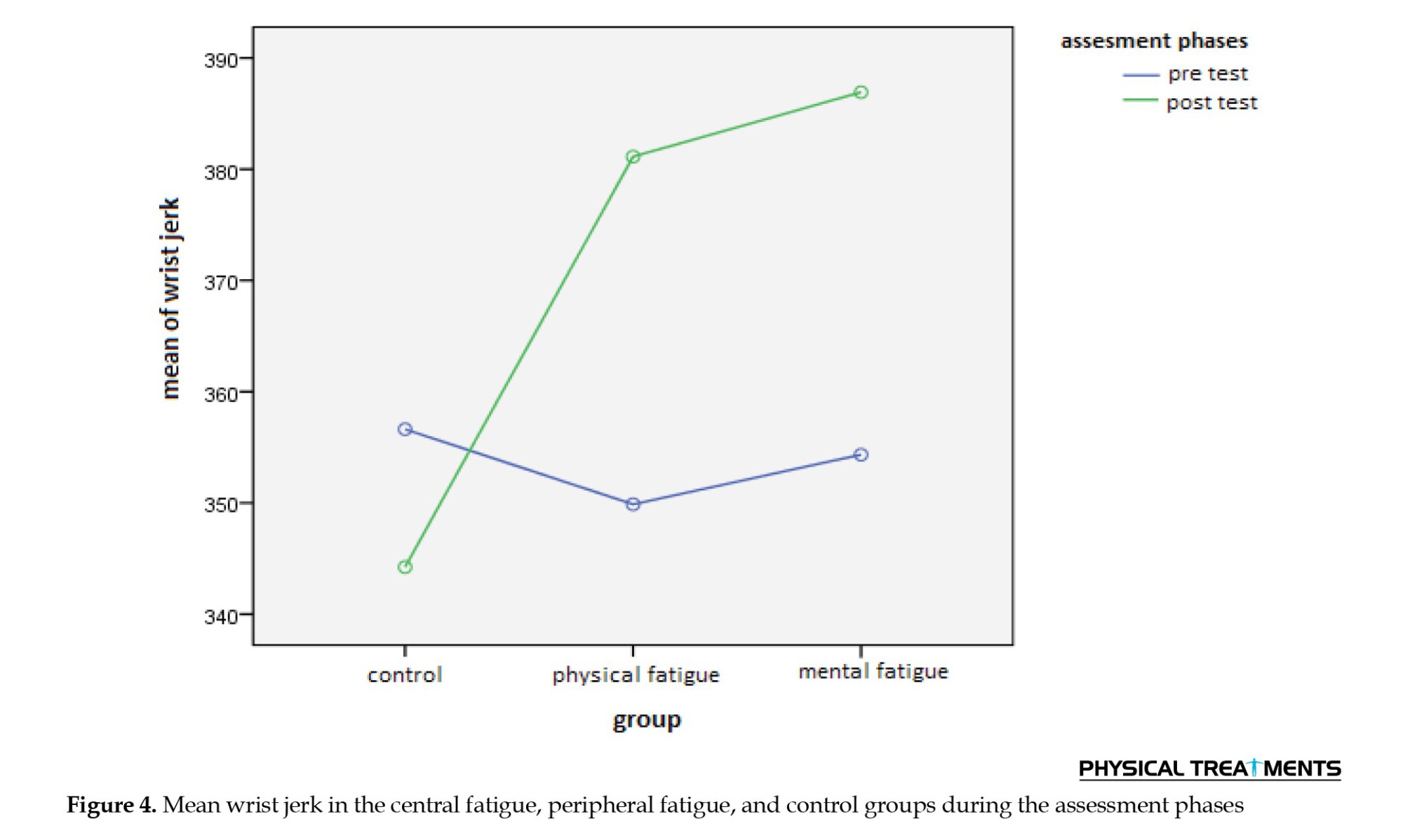
No significant difference was found between the central fatigue group and the peripheral fatigue group (P>0.05). Post-test mean comparisons revealed that the control group had a lower mean jerk (M=344.24) than both the central fatigue group (M=386.92) and the peripheral fatigue group (M=381.13). These results suggest smoother performance (less jerk) in the control group during the post-test.
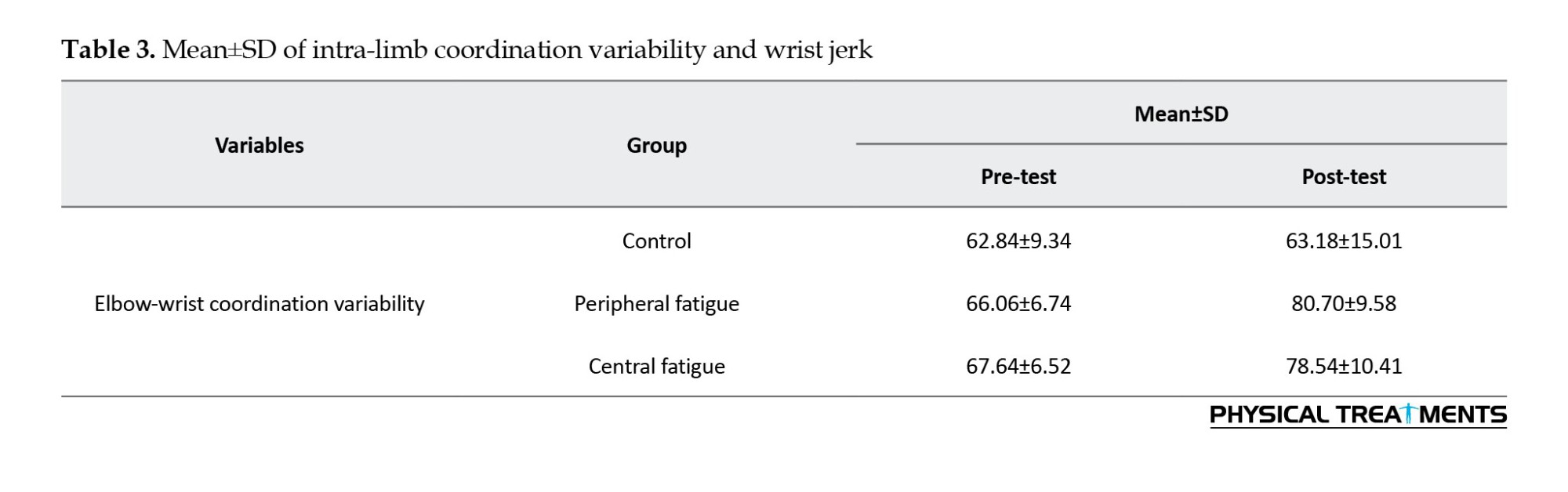
The Mean±SD of intra-limb coordination variability are presented in Table 3. The findings presented in Table 3 show that in the post-test, the mean variability of intra-limb elbow-wrist coordination in the peripheral fatigue and central fatigue groups was higher than in the control group. The results of one-way ANOVA showed that there was no significant difference between the mean variability of the elbow-wrist movement coordination pattern among the groups in the pre-test phase (F(2, 27)=1.02, P=0.37).
To compare the mean variability of the elbow-wrist movement coordination pattern, a mixed ANOVA with repeated measures (2×3) and Bonferroni post hoc test was used (Table 4).
The results of the repeated measures ANOVA in Table 4 indicated a significant main effect of the assessment phase (P=0.001). Examination of the means revealed that the variability of the elbow-wrist movement coordination pattern was higher in the post-test (M=74.14) than in the pre-test (M=65.51). The main effect of group was also significant (P=0.026). Bonferroni post hoc tests showed a significant difference in the mean variability of the elbow-wrist coordination pattern between the control group and both the peripheral and central fatigue groups (P<0.05). However, no significant difference was found between the central fatigue and peripheral fatigue groups (P>0.05). The mean variability in the control group (M=63.01) was lower than in both the central fatigue (M=73.09) and peripheral fatigue (M=73.38) groups.
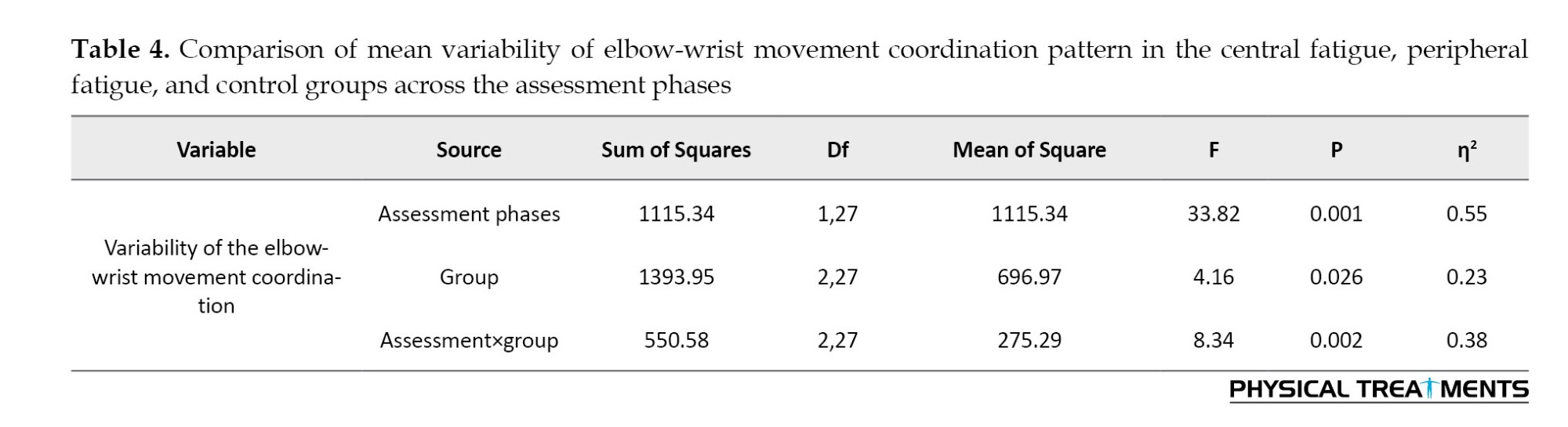
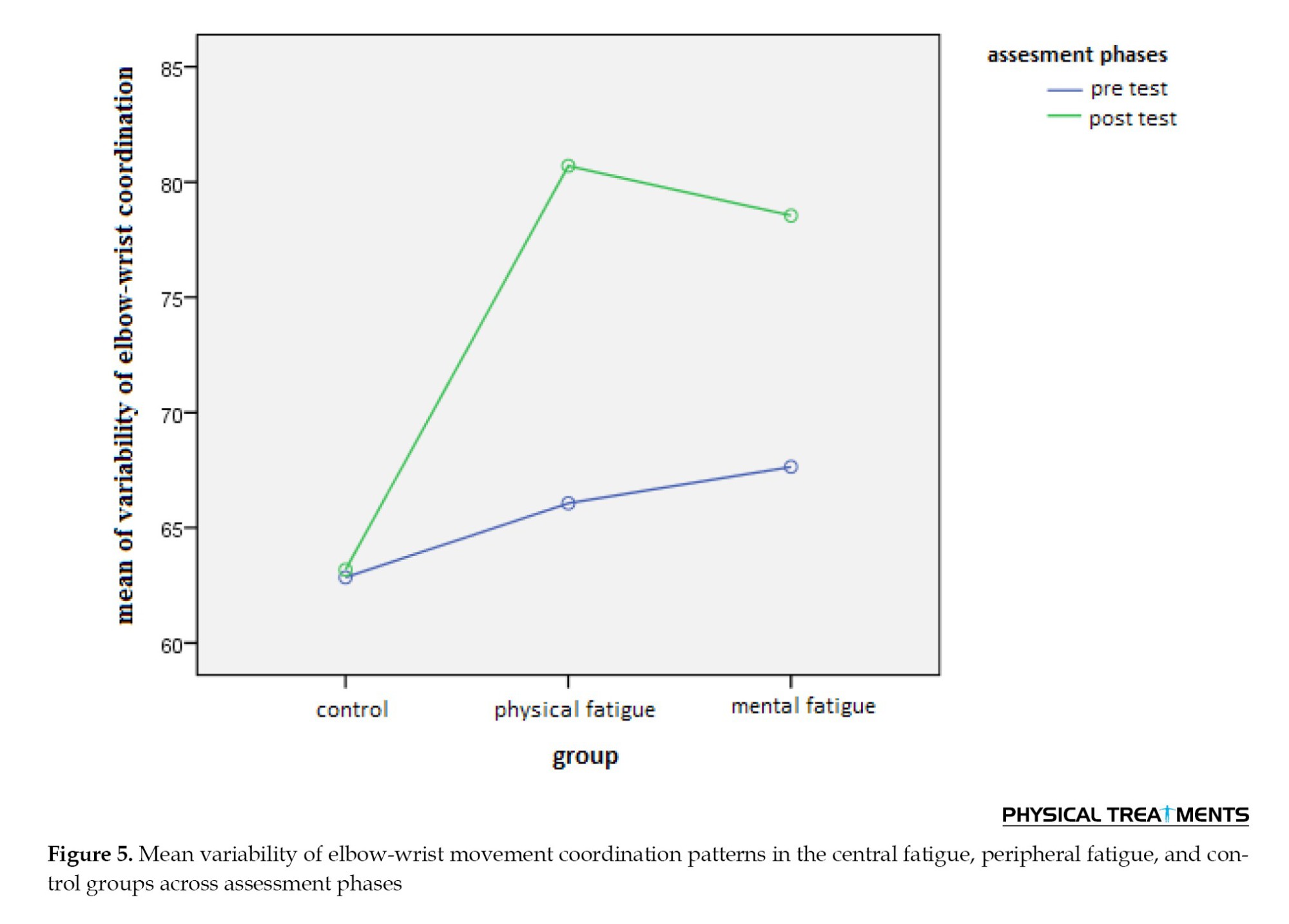
The interaction effect between the assessment phase and group was significant (P=0.002). interactive Figure 5 and pairwise comparisons demonstrated a significant difference in movement coordination variability between the control group and the peripheral/central fatigue groups (P<0.05), but no significant difference was found between the central and peripheral fatigue groups (P>0.05).
Post-test mean comparisons further indicated that movement coordination variability in the control group (M=63.18) was lower than in the central fatigue (M=78.54) and peripheral fatigue (M=80.70) groups.
Discussion
The primary objective of this investigation was to compare the effects of central and peripheral fatigue on movement variability and movement smoothness in golf swing performance. The results indicated that participants in the control group exhibited lower (better) mean variability in motor coordination patterns and better mean movement smoothness compared to the central fatigue and peripheral fatigue groups.
In the peripheral fatigue group, there was no discernible difference in the average variability of the motor coordination pattern between the memorization and pre-test stages. In contrast, the central fatigue group showed a significant difference in mean variability between these stages, with higher (worse) variability during the memory test compared to the pre-test. Overall, the central fatigue group demonstrated the greatest variability in coordination patterns, while the non-intervention group exhibited the least. The peripheral fatigue group displayed higher variability than the control group but lower variability than the central fatigue group.
The results of the present study demonstrate that central fatigue increases movement variability and reduces movement smoothness. Researchers have established central fatigue as a key factor negatively impacting kinematics and athletic performance. Notably, mental exhaustion has been directly associated with declines in hand and foot coordination. Coordinated movements, defined as sequences of voluntary actions synchronized at the cortical level [20], are especially vulnerable to such fatigue. These findings are consistent with those of Smith et al. [21] and Marcora et al. [22] all of whom observed the detrimental effects of central fatigue on motor control and coordination. Mentally fatigued individuals display reduced accuracy, timing, and stability in tasks requiring fine motor skills, such as dynamic balance and hand-eye coordination. This impairment is often accompanied by increased postural fluctuations and diminished stability, indicating that central fatigue hinders the ability to sustain optimal movement patterns. Collectively, these findings reinforce the idea that central fatigue disrupts the integration of sensory and motor processes, resulting in less efficient movement strategies [22].
Zahiri et al. [20] highlight that central fatigue adversely affects behavior and attention by disrupting the central nervous system. Additionally, central fatigue modifies muscle activation patterns, especially during tasks demanding sustained attention and precise motor control. Smith et al. found that central fatigue leads to delayed muscle onset and diminished coordination between agonist and antagonist muscles during repetitive tasks. This decline in muscle coordination not only compromises movement efficiency but also heightens the risk of compensatory biomechanical patterns, which may contribute to musculoskeletal strain and injury over time [21]. At the neurophysiological level, central fatigue is associated with reduced activity in the prefrontal cortex, a brain region critical for decision-making, attention, and motor planning. Marcora et al. demonstrated that central fatigue disrupts cognitive-motor integration, leading to slower reaction times and poorer motor accuracy. This suggests that the brain’s ability to process sensory information and execute coordinated movements is impaired under mental fatigue. Additionally, the depletion of monoaminergic neurotransmitters (e.g. dopamine and norepinephrine), which play a key role in maintaining motivation and motor performance, may exacerbate these effects [22]. Similarly, Lederman et al. [23] linked mental fatigue to reduced muscle response, diminished dynamic joint stability, and decreased excitability of corticomotor neurons, ultimately impairing balance.
Building on this, Martin et al. [24] further elaborated that mental fatigue—resulting from adenosine accumulation in the brain and resistance to increased effort—leads to feelings of exhaustion and low energy. This disruption in neuromuscular control, potentially due to perceived weakness and weariness, delays neuromuscular activation, increasing torque and shear forces and thereby compromising joint stability [6]. Thorndike’s theory of central fatigue mechanics posits that prolonged mental work gradually diminishes the productivity of mental functions. Individuals engaged in cognitively demanding tasks often experience prolonged mental exertion, reducing sustained attention, productivity, and limiting adaptability in unpredictable situations [25]. Supporting this, Skala and Zamkova [26] demonstrated that central fatigue induced by at least 30 minutes of the Stroop color-word task and smartphone use negatively impacts cognitive performance in sports tests, such as the football acceptance test. They also noted that both central and peripheral fatigue can alter players› attention and perception levels.
However, not all findings align with these observations. For instance, De Vleeschouwer et al. [27] reported that central fatigue does not affect lower-limb kinematics during lateral landing jumps. They suggested that the primary impact of central fatigue on performance stems from individuals› impaired ability to allocate attention effectively. According to the parallel information processing model [28], focusing on fatigue sensations prevents optimal task performance. Easterbrook’s perceptual narrowing theory further supports this, demonstrating that central fatigue restricts attentional focus, thereby impairing performance [11]. Additionally, Hasan et al. [11] found that central fatigue adversely affects both active and passive knee proprioception and balance, disrupting active proprioceptive sensation. Given that central fatigue is linked to central nervous system dysfunction, these findings are validated, as coordinated movements rely on sequences of voluntary actions regulated at the cortical level [29].
On the other hand, this research demonstrated that peripheral fatigue is one of the factors influencing coordination pattern variability. This finding aligns with the results of Cowley and Geis [30]. Their study revealed that the primary manifestations of shoulder fatigue include increased elbow flexion, decreased arm height, and greater left trunk angle and angular velocity. Furthermore, they found that motion variability increased more in proximal joints than in distal joints following both fatigue protocols, with a more pronounced effect after proximal fatigue [30]. Additionally, researchers have examined the impact of fatigue on shooting kinematics, indicating that when players shoot under moderate to severe fatigue, significant changes occur in arm and shoulder positioning [31, 32].
Uygur et al. found that fatigue does not significantly influence the selected movement variables of the free throw. In contrast, other researchers have examined the effects of lower limb fatigue on gait parameters in healthy young individuals [31]. Their observations during fatigue tests revealed a notable reduction in the center of mass of the knee joint and peak point, increased knee flexion, and decreased ankle dorsiflexion during the heel strike phase of the gait cycle [33].
According to Afhami et al. athletes exhibit greater neck angle reconstruction errors compared to non-athletes due to neck muscle fatigue [34]. Gao et al. found that lower limb symmetry remained unaffected after running under fatigue conditions, with similar levels of peripheral-central fatigue observed in both limbs [35]. Movement variability reflects neuromuscular control capacity, and fatigue serves as a key factor increasing variability in subsequent efforts. This increased variability indicates reduced motor control, arising from movement noise, disrupted ion channel and synaptic function, and neural instability [36].
Fatigue directly impacts muscles and their contraction mechanisms while progressively decreasing involuntary muscle activation. It elevates the discharge threshold of muscle spindles, disrupts alpha-gamma coactivation, and causes sensory signals to shift to alpha motor neurons. This neural adaptation impairs the muscle-joint coordination needed for proper protective function. Such changes may lead to altered neuromuscular control in the lower limbs and modified afferent input from peripheral receptors [32]. Ultimately, fatigue-induced modifications in afferent signals from lower limb muscle receptors can diminish athletic performance and elevate injury risk [37, 38].
Another key finding of this study indicates that jerk, as an indicator of movement smoothness, is influenced by multiple factors, including fatigue and skill level. According to motor control theories, the central nervous system optimizes movements to minimize jerk, thereby producing smoother and more efficient motion patterns [39]. This optimization is particularly pronounced in skilled performers, where lower jerk values reflect superior neuromuscular coordination and refined motor control (Harris & Wolpert, 1998) [40]. However, fatigue—whether central or peripheral—can impair these control mechanisms. Peripheral fatigue compromises muscle activation and joint stability, while central fatigue disrupts cognitive-motor integration and elevates movement variability [40]. These combined effects suggest that fatigue degrades motor performance by increasing jerk and reducing movement smoothness, consequently heightening injury risk during dynamic movements. These findings underscore the critical importance of fatigue management in sports and complex motor tasks. Implementing targeted strategies, such as enhanced physical conditioning, cognitive training, and optimized recovery protocols, can help counteract fatigue’s detrimental effects on movement quality and coordination [40, 41].
However, several limitations of this study must be acknowledged to properly contextualize the findings. While this research provides valuable insights into how central and peripheral fatigue affect motor coordination during golf swings, certain constraints should be noted. First, the study’s sample consisted exclusively of young, healthy female students, which may limit the generalizability of the results to other populations, such as male golfers, older individuals, or professional athletes. Future studies should incorporate more diverse participant groups to verify these findings across different demographics. Second, the laboratory setting, while controlled, may not accurately reflect the dynamic conditions of actual golf play. Environmental factors, including variable weather conditions, uneven terrain, and competitive pressure—all known to influence fatigue development and motor coordination—were not replicated in this experimental setup. These real-world variables could potentially modify the observed relationships between fatigue and swing coordination patterns.
To improve ecological validity, future research should incorporate field-based experiments that more accurately simulate the complex demands of actual golf performance. Third, the current study’s fatigue protocols may not precisely represent the specific challenges of competitive golf. While the Stroop task served as our central fatigue induction method (a well-established cognitive test), it may not fully capture the mental demands of an actual golf game. Similarly, the plank-based peripheral fatigue protocol might not adequately reproduce the exact muscular fatigue patterns generated during golf swings. Future investigations should develop sport-specific fatigue protocols that better replicate golf’s unique physical and cognitive requirements.
Additionally, while movement variability served as our primary outcome measure, we acknowledge that increased variability does not necessarily indicate impaired performance. In certain contexts, greater variability may demonstrate beneficial motor adaptability and system resilience. Addressing these limitations in subsequent studies will advance our understanding of how central and peripheral fatigue affect coordination patterns in golf and other precision sports.
Conclusion
The findings of this study reveal that the motor coordination pattern variability in the muscular fatigue group was significantly greater than that of the control group but lower than that of the central fatigue group. Notably, the central fatigue group demonstrated the highest coordination variability, underscoring the profound influence of central fatigue on motor control. This research provides the first simultaneous investigation of both peripheral and central fatigue effects on golf swing mechanics, suggesting the need for additional studies to examine these relationships across different protocols and sporting contexts.
Based on these results, we recommend that coaches and instructors employ targeted strategies to address both central and peripheral fatigue. For mental fatigue reduction, interventions, such as meditation, mental imagery, and mindfulness training may prove beneficial. Concurrently, enhancing physical conditioning can raise the threshold for peripheral fatigue onset. By implementing comprehensive fatigue management programs that optimize motivation and recovery, practitioners can help athletes sustain peak performance while minimizing injury risk.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Alzahra University, Tehran, Iran (Code: SSRI.REC-2211-1962). All ethical principles were strictly followed in this study. Participants were fully informed about the research objectives and procedures. They received assurances regarding data confidentiality and retained the right to withdraw from the study at any time. Participants were also informed that study results would be made available to them upon request. Written informed consent was obtained from all participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge all the participants for their involvement in this study.
References
Motor activity constitutes a fundamental aspect of human movement, shaped by individual capacity, experience, and environmental adaptations. In precision-based sports, such as golf, where coordination and biomechanical efficiency are paramount, fatigue represents a critical factor influencing performance. Motor coordination relies on neuromuscular control to facilitate smooth and efficient movements [1]. However, fatigue disrupts this process, inducing variations in movement patterns, increasing error rates, and heightening the risk of injury [2].
Arises from prolonged physical exertion, leading to a decline in the neuromuscular system’s capacity for force production and inter-muscular synchronization [3]. According to Tornero-Aguilera et al. central and peripheral fatigue have been broadly explained in exercise physiology literature [4]. Furthermore, Izadi et al. reported that both central and peripheral fatigue negatively affect movement coordination and accuracy in handball players [5]. Similarly, Ortiz et al. emphasize that fatigue-induced alterations in motor coordination negatively affect joint stability, particularly in sports requiring repetitive movement patterns [6]. Given these implications, it is hypothesized that muscular fatigue detrimentally affects motor coordination in golf, increasing variability in movement execution.
Conversely, central fatigue results from sustained cognitive exertion, impairing central nervous system activity and potentially disrupting motor control and proprioception [7, 8]. Tornero-Aguilera et al. suggest that central fatigue disrupts cortical processing, leading to prolonged reaction times and diminished movement accuracy [4]. Studies in other sports, such as soccer, show that central fatigue negatively affects passing decision-making and cognitive-motor integration [9]. Similarly, Smith et al. demonstrate that central fatigue significantly compromises decision-making and motor execution in precision-dependent sports, such as golf [10]. Neuroscientific research indicates that mental exhaustion alters neuromuscular responses, disrupts proprioception, and hinders motor planning due to its influence on the frontal cortex, ultimately impairing performance [11].
Understanding the effects of fatigue is especially critical in sports requiring both physical endurance and cognitive acuity. Golf presents a unique challenge, demanding precise motor execution, sustained concentration, and biomechanical efficiency [12-14]. Unlike more physically intensive sports, where muscular endurance is paramount, golf relies on maintaining stable and controlled movement patterns over extended periods [13]. Competitive golf requires substantial cognitive engagement, motor coordination, and endurance [13]. The impact of fatigue on golf performance extends beyond muscular exhaustion, involving complex interactions between neuromuscular and cognitive functions.
Empirical evidence indicates that fatigue significantly impacts the biomechanics of the golf swing [12]. Hakukawa et al. found that fatigue-induced changes in trunk and lower-limb kinematics directly affect swing mechanics and shot accuracy [14]. Research on movement analysis techniques highlights the reliability of motion tracking systems for assessing motor performance under fatigue [15]. Additionally, mental fatigue has been shown to negatively affect functional performance tests, impairing motor planning, decision-making, and execution [16]. Similarly, Gebel et al. reported that postural control declines under fatigue, leading to increased movement variability and reduced shot precision in golfers [17].
Central fatigue also impairs performance, with golfers exhibiting slower reaction times, decreased movement accuracy, and greater kinematic variability [13]. Studies indicate that central fatigue diminishes movement automaticity, forcing athletes to rely more on conscious control, which paradoxically increases variability and reduces performance consistency [8]. Athletes’ perceived exertion, measured via the Borg RPE scale, correlates with both physical and mental fatigue, affecting performance outcomes [18]. Heart rate variability has also been shown to reflect fatigue levels and predict decrements in motor control [19]. Central and peripheral fatigue together impair coordination and timing, further compromising precision-dependent tasks [20]. Neuromuscular rehabilitation research highlights that fatigued individuals exhibit altered motor recruitment patterns and delayed response times [21]. Mental fatigue specifically impairs endurance and cognitive function, reducing the ability to maintain optimal performance under prolonged cognitive load [22]. Cognitive exhaustion has been demonstrated to decrease cognitive flexibility, thereby affecting decision-making and motor planning in sports contexts [23]. Collectively, these effects result in suboptimal motor planning and execution [24].
Addressing this gap, this study aimed to optimize training protocols, fatigue management strategies, and injury prevention measures in golf. Central fatigue is hypothesized to have a more pronounced effect due to its direct influence on cognitive processing and neuromuscular responses [22]. Understanding the distinct effects of central and peripheral fatigue on golf performance could offer valuable insights for enhancing endurance strategies and mitigating performance decline in athletes, ultimately improving both athletic performance and injury prevention [14].
Given these considerations, the present study systematically compared the effects of central and peripheral fatigue on motor coordination in the golf swing to determine which type of fatigue more significantly impacts performance. Notably, fatigue—whether muscular or mental—disrupts motor regulation by altering control strategies, increasing movement inconsistencies, and reducing accuracy. Given the established influence of fatigue on movement variability and smoothness, the study hypothesized that:
1) Both types of fatigue will negatively affect movement variability and smoothness; 2) central fatigue will induce greater variability in motor coordination patterns than peripheral fatigue, as it more substantially impairs central motor control mechanisms [10].
Materials and Methods
This study employed an applied, semi-experimental research design, utilizing pre-test and post-test procedures across three groups: Central fatigue, peripheral fatigue, and a non-intervention group. The statistical population consisted of female students. G*Power software, version 5.3.3 was used to estimate the sample size, with a test power of 0.8, an effect size of 0.8, and an alpha level of 0.05. Participants were selected using criterion-based purposive sampling.
The final sample included 30 young, healthy female sports science students, aged 20–35 years. Eligibility criteria required participants to have no prior experience in golf, be right-handed, have normal or corrected-to-normal vision, be free of underlying health conditions, abstain from regular alcohol or tobacco use, and be in good physical and mental health. During the familiarization session, all testing methods and equipment were explained. To ensure consistency, participants were instructed to get adequate rest (a minimum of eight hours), avoid caffeine, alcohol, heavy exercise, and mentally demanding tasks 24 hours before testing, and consume a nutritious meal approximately 1.5 hours before each session.
Testing sessions were conducted at least 72 hours apart. Individuals were excluded if they had a history of neurological disorders, took medications affecting cortical function, or sustained lower-limb musculoskeletal injuries (e.g. ankle sprains) within the past six months. Written informed consent was obtained after full disclosure of the study’s objectives and protocols.
Procedure
Initially, the subjects hit a 2-meter target three times to warm up and become comfortable with the technique. Each participant was then given ten attempts to hit the target to complete the pre-test. A total of 48 optical markers were attached to the skin, and eight motion analysis cameras with a sampling rate of 240 Hz recorded the subject’s movements during the stroke (Figures 1 and 2). Participants were instructed to stop the ball as close to the target as possible. Twenty-four hours later, the fatigue task was administered first to the peripheral fatigue group. To prevent physical injury, each subject followed a 10-minute protocol involving stretching and dynamic movements, such as jumping and squatting (including sleeping, sitting, standing, and jumping). Subsequently, a modified Borg scale plank exercise was performed for 15 minutes [14].

A score of more than 17 on this scale indicated that the subjects felt fatigued and weak. During the exercises, a heart rate monitor was used to track participant activity and heart rate. The participants’ heart rates increased to 70% of their maximum, and they continued exercising until exhaustion. After completing the fatigue protocol, the subjects took ten golf shots toward the target.

In the central fatigue group, the central fatigue intervention was applied first. Participants in this group first completed the visual analog scale (VAS) to measure their baseline central fatigue levels. They then performed the Stroop task for 45 minutes (Figure 3). After the Stroop task, they completed the VAS again. If participants scored at least 50 points on the VAS, it indicated that they had reached the desired level of central fatigue [10]. In such cases, they continued the Stroop task while completing the VAS every 10 minutes until the intended level of central fatigue was achieved.

In the non-intervention group, participants performed a block of ten golf swings during both the pre-test and retention stages. They rated their perceived exertion using a calibrated Borg scale (ranging from 6 to 20.23) at the start and end of each session. Perceived exertion was defined as the conscious sensation of how hard or strenuous the activity felt [18].
Motion analysis system to record changes in body angles
A motion capture system consisting of eight infrared cameras (AM6110, Bertec, Columbus, OH, USA; frequency: 600 Hz) was used, with data sampled at 250 Hz (Oqus, Qualisys, Sweden). A total of 48 reflective markers were placed on standardized bony landmarks. Marker motion was recorded using Qualisys Track Manager software, version 2.7. Kinematic data and swing speed were calculated using Visual3D (C-Motion, Rockville, MD, USA) [14].
Subjective level of central fatigue
Participants rated their perceived fatigue levels on the VAS, ranging from 0 (not physically/mentally fatigued at all) to 10 (extremely physically/mentally fatigued), to assess subjective levels of physical and mental fatigue (PF/MF) [16]. Subjective measures of central fatigue were taken before and after the fatigue protocols, as previously documented by Verschueren et al. [16]. To confirm central fatigue induction, participants rated their perceived fatigue on the VAS (0–10) before and after the task. An increase in perceived fatigue post-intervention verified central fatigue establishment. This method aligns with prior research indicating that a post-task VAS score increase of at least 3 points reliably indicates central fatigue [16].
All participants in the central fatigue group successfully completed the test. To induce central fatigue, players performed a 30-minute computerized Stroop color-word task, which has been widely used in sports research due to its effectiveness in inducing central fatigue [17]. In this cognitive task, four words (red, blue, green, yellow) appeared individually on a grey-background computer screen. Participants responded by pressing a key corresponding to the word’s color rather than its meaning (e.g. if red appeared in blue, the correct response was the blue key).
To increase difficulty and attention demands, an exception was applied: if the word’s color was red, participants had to press the key matching the word’s meaning (e.g. if green appeared in red, the correct response was the green key).
The task consisted of 50% congruent trials (word and color matching) and 50% incongruent trials. Each word appeared for 1000 ms, followed by a 1000-ms black screen before the next word appeared, resulting in a new stimulus every 2000 ms (900 total stimuli). Incorrect or delayed responses (>1500 ms) triggered a beep to prompt faster or more accurate responses. To enhance motivation, participants were challenged to complete as many correct responses as possible within the 30-minute period while competing for speed and accuracy against others [17].
Rating of perceived exertion
Perceived exertion was recorded using the Borg scale (ranging from 6 to 20) at standardized intervals, both at the start and end of each game [18]. Perceived exertion reflects the subjective cognitive appraisal of physical effort intensity during activity [19].
Peripheral fatigue task
Peripheral fatigue was induced through progressive plank exercises under the researcher’s supervision [14]. The plank is an isometric exercise that promotes muscular fatigue via sustained contraction, resulting in a progressive decline in neuromuscular efficiency—consistent with the study’s objective of examining fatigue’s impact on motor coordination [20]. As a widely used whole-body exercise targeting the trunk, the plank is considered a high-intensity workout.
The forearm plank was performed in accordance with ACSM guidelines: “Body weight supported on forearms and toes, with scapulae protracted and pelvis posteriorly tilted.” To ensure controlled and consistent fatigue induction, participants maintained the plank position until reaching a perceived exertion level of 17 or higher on the modified Borg Scale (indicating “extremely difficult” exertion). Upon reaching this threshold, participants were instructed to stop. Following the plank task, a 30-second rest period was provided before participants performed the golf swing task. This approach standardized peripheral fatigue induction while minimizing the risk of injury and maintaining control over task intensity [14].
Heart rate monitoring
To monitor heart rate and confirm that participants reached approximately 90% of their maximal theoretical heart rate during the fatigue session—equivalent to the average heart rate observed during a badminton match—each participant wore a Polar RS400 running computer [19].
Statistical methods
The Shapiro-Wilk test was used to check the normality of data distribution, and Levene’s test was used to assess the homogeneity of variances. To investigate the main effects of fatigue type (between-group differences) and assessment phase (within-group differences), as well as the interaction effect of fatigue type with assessment phase, a mixed ANOVA with repeated measures (2×3) and Bonferroni’s post hoc test was used. Data analysis was conducted using SPSS software, version 20.
Results
The Mean±SD of wrist jerk are presented in Table 1. In the post-test, the mean wrist jerk in the peripheral fatigue and central fatigue groups was higher than in the control group. A one-way analysis of variance revealed no significant difference in the mean jerk movements between the groups during the pre-test phase (F(2, 27)=0.12, P=0.87).To compare the mean wrist jerk movements, a Mixed ANOVA with repeated measures (2×3) was conducted, followed by a Bonferroni post hoc test (Table 2).

The results of the repeated measures ANOVA (Table 2) revealed a significant main effect of the assessment phase (P=0.001). Examination of the means showed that the mean jerk of movement in the post-test (M=370.76) was higher than in the pre-test (M=353.61). The main effect of group was not statistically significant (P=0.26). However, the interaction effect of assessment phase×group was significant (P=0.001). Pairwise comparisons (see interactive Figure 4) indicated a significant difference in the mean jerk of movement between the control group and both the central fatigue and peripheral fatigue groups (P<0.05).


No significant difference was found between the central fatigue group and the peripheral fatigue group (P>0.05). Post-test mean comparisons revealed that the control group had a lower mean jerk (M=344.24) than both the central fatigue group (M=386.92) and the peripheral fatigue group (M=381.13). These results suggest smoother performance (less jerk) in the control group during the post-test.
The Mean±SD of intra-limb coordination variability are presented in Table 3. The findings presented in Table 3 show that in the post-test, the mean variability of intra-limb elbow-wrist coordination in the peripheral fatigue and central fatigue groups was higher than in the control group. The results of one-way ANOVA showed that there was no significant difference between the mean variability of the elbow-wrist movement coordination pattern among the groups in the pre-test phase (F(2, 27)=1.02, P=0.37).

To compare the mean variability of the elbow-wrist movement coordination pattern, a mixed ANOVA with repeated measures (2×3) and Bonferroni post hoc test was used (Table 4).
The results of the repeated measures ANOVA in Table 4 indicated a significant main effect of the assessment phase (P=0.001). Examination of the means revealed that the variability of the elbow-wrist movement coordination pattern was higher in the post-test (M=74.14) than in the pre-test (M=65.51). The main effect of group was also significant (P=0.026). Bonferroni post hoc tests showed a significant difference in the mean variability of the elbow-wrist coordination pattern between the control group and both the peripheral and central fatigue groups (P<0.05). However, no significant difference was found between the central fatigue and peripheral fatigue groups (P>0.05). The mean variability in the control group (M=63.01) was lower than in both the central fatigue (M=73.09) and peripheral fatigue (M=73.38) groups.

The interaction effect between the assessment phase and group was significant (P=0.002). interactive Figure 5 and pairwise comparisons demonstrated a significant difference in movement coordination variability between the control group and the peripheral/central fatigue groups (P<0.05), but no significant difference was found between the central and peripheral fatigue groups (P>0.05).
Post-test mean comparisons further indicated that movement coordination variability in the control group (M=63.18) was lower than in the central fatigue (M=78.54) and peripheral fatigue (M=80.70) groups.

Discussion
The primary objective of this investigation was to compare the effects of central and peripheral fatigue on movement variability and movement smoothness in golf swing performance. The results indicated that participants in the control group exhibited lower (better) mean variability in motor coordination patterns and better mean movement smoothness compared to the central fatigue and peripheral fatigue groups.
In the peripheral fatigue group, there was no discernible difference in the average variability of the motor coordination pattern between the memorization and pre-test stages. In contrast, the central fatigue group showed a significant difference in mean variability between these stages, with higher (worse) variability during the memory test compared to the pre-test. Overall, the central fatigue group demonstrated the greatest variability in coordination patterns, while the non-intervention group exhibited the least. The peripheral fatigue group displayed higher variability than the control group but lower variability than the central fatigue group.
The results of the present study demonstrate that central fatigue increases movement variability and reduces movement smoothness. Researchers have established central fatigue as a key factor negatively impacting kinematics and athletic performance. Notably, mental exhaustion has been directly associated with declines in hand and foot coordination. Coordinated movements, defined as sequences of voluntary actions synchronized at the cortical level [20], are especially vulnerable to such fatigue. These findings are consistent with those of Smith et al. [21] and Marcora et al. [22] all of whom observed the detrimental effects of central fatigue on motor control and coordination. Mentally fatigued individuals display reduced accuracy, timing, and stability in tasks requiring fine motor skills, such as dynamic balance and hand-eye coordination. This impairment is often accompanied by increased postural fluctuations and diminished stability, indicating that central fatigue hinders the ability to sustain optimal movement patterns. Collectively, these findings reinforce the idea that central fatigue disrupts the integration of sensory and motor processes, resulting in less efficient movement strategies [22].
Zahiri et al. [20] highlight that central fatigue adversely affects behavior and attention by disrupting the central nervous system. Additionally, central fatigue modifies muscle activation patterns, especially during tasks demanding sustained attention and precise motor control. Smith et al. found that central fatigue leads to delayed muscle onset and diminished coordination between agonist and antagonist muscles during repetitive tasks. This decline in muscle coordination not only compromises movement efficiency but also heightens the risk of compensatory biomechanical patterns, which may contribute to musculoskeletal strain and injury over time [21]. At the neurophysiological level, central fatigue is associated with reduced activity in the prefrontal cortex, a brain region critical for decision-making, attention, and motor planning. Marcora et al. demonstrated that central fatigue disrupts cognitive-motor integration, leading to slower reaction times and poorer motor accuracy. This suggests that the brain’s ability to process sensory information and execute coordinated movements is impaired under mental fatigue. Additionally, the depletion of monoaminergic neurotransmitters (e.g. dopamine and norepinephrine), which play a key role in maintaining motivation and motor performance, may exacerbate these effects [22]. Similarly, Lederman et al. [23] linked mental fatigue to reduced muscle response, diminished dynamic joint stability, and decreased excitability of corticomotor neurons, ultimately impairing balance.
Building on this, Martin et al. [24] further elaborated that mental fatigue—resulting from adenosine accumulation in the brain and resistance to increased effort—leads to feelings of exhaustion and low energy. This disruption in neuromuscular control, potentially due to perceived weakness and weariness, delays neuromuscular activation, increasing torque and shear forces and thereby compromising joint stability [6]. Thorndike’s theory of central fatigue mechanics posits that prolonged mental work gradually diminishes the productivity of mental functions. Individuals engaged in cognitively demanding tasks often experience prolonged mental exertion, reducing sustained attention, productivity, and limiting adaptability in unpredictable situations [25]. Supporting this, Skala and Zamkova [26] demonstrated that central fatigue induced by at least 30 minutes of the Stroop color-word task and smartphone use negatively impacts cognitive performance in sports tests, such as the football acceptance test. They also noted that both central and peripheral fatigue can alter players› attention and perception levels.
However, not all findings align with these observations. For instance, De Vleeschouwer et al. [27] reported that central fatigue does not affect lower-limb kinematics during lateral landing jumps. They suggested that the primary impact of central fatigue on performance stems from individuals› impaired ability to allocate attention effectively. According to the parallel information processing model [28], focusing on fatigue sensations prevents optimal task performance. Easterbrook’s perceptual narrowing theory further supports this, demonstrating that central fatigue restricts attentional focus, thereby impairing performance [11]. Additionally, Hasan et al. [11] found that central fatigue adversely affects both active and passive knee proprioception and balance, disrupting active proprioceptive sensation. Given that central fatigue is linked to central nervous system dysfunction, these findings are validated, as coordinated movements rely on sequences of voluntary actions regulated at the cortical level [29].
On the other hand, this research demonstrated that peripheral fatigue is one of the factors influencing coordination pattern variability. This finding aligns with the results of Cowley and Geis [30]. Their study revealed that the primary manifestations of shoulder fatigue include increased elbow flexion, decreased arm height, and greater left trunk angle and angular velocity. Furthermore, they found that motion variability increased more in proximal joints than in distal joints following both fatigue protocols, with a more pronounced effect after proximal fatigue [30]. Additionally, researchers have examined the impact of fatigue on shooting kinematics, indicating that when players shoot under moderate to severe fatigue, significant changes occur in arm and shoulder positioning [31, 32].
Uygur et al. found that fatigue does not significantly influence the selected movement variables of the free throw. In contrast, other researchers have examined the effects of lower limb fatigue on gait parameters in healthy young individuals [31]. Their observations during fatigue tests revealed a notable reduction in the center of mass of the knee joint and peak point, increased knee flexion, and decreased ankle dorsiflexion during the heel strike phase of the gait cycle [33].
According to Afhami et al. athletes exhibit greater neck angle reconstruction errors compared to non-athletes due to neck muscle fatigue [34]. Gao et al. found that lower limb symmetry remained unaffected after running under fatigue conditions, with similar levels of peripheral-central fatigue observed in both limbs [35]. Movement variability reflects neuromuscular control capacity, and fatigue serves as a key factor increasing variability in subsequent efforts. This increased variability indicates reduced motor control, arising from movement noise, disrupted ion channel and synaptic function, and neural instability [36].
Fatigue directly impacts muscles and their contraction mechanisms while progressively decreasing involuntary muscle activation. It elevates the discharge threshold of muscle spindles, disrupts alpha-gamma coactivation, and causes sensory signals to shift to alpha motor neurons. This neural adaptation impairs the muscle-joint coordination needed for proper protective function. Such changes may lead to altered neuromuscular control in the lower limbs and modified afferent input from peripheral receptors [32]. Ultimately, fatigue-induced modifications in afferent signals from lower limb muscle receptors can diminish athletic performance and elevate injury risk [37, 38].
Another key finding of this study indicates that jerk, as an indicator of movement smoothness, is influenced by multiple factors, including fatigue and skill level. According to motor control theories, the central nervous system optimizes movements to minimize jerk, thereby producing smoother and more efficient motion patterns [39]. This optimization is particularly pronounced in skilled performers, where lower jerk values reflect superior neuromuscular coordination and refined motor control (Harris & Wolpert, 1998) [40]. However, fatigue—whether central or peripheral—can impair these control mechanisms. Peripheral fatigue compromises muscle activation and joint stability, while central fatigue disrupts cognitive-motor integration and elevates movement variability [40]. These combined effects suggest that fatigue degrades motor performance by increasing jerk and reducing movement smoothness, consequently heightening injury risk during dynamic movements. These findings underscore the critical importance of fatigue management in sports and complex motor tasks. Implementing targeted strategies, such as enhanced physical conditioning, cognitive training, and optimized recovery protocols, can help counteract fatigue’s detrimental effects on movement quality and coordination [40, 41].
However, several limitations of this study must be acknowledged to properly contextualize the findings. While this research provides valuable insights into how central and peripheral fatigue affect motor coordination during golf swings, certain constraints should be noted. First, the study’s sample consisted exclusively of young, healthy female students, which may limit the generalizability of the results to other populations, such as male golfers, older individuals, or professional athletes. Future studies should incorporate more diverse participant groups to verify these findings across different demographics. Second, the laboratory setting, while controlled, may not accurately reflect the dynamic conditions of actual golf play. Environmental factors, including variable weather conditions, uneven terrain, and competitive pressure—all known to influence fatigue development and motor coordination—were not replicated in this experimental setup. These real-world variables could potentially modify the observed relationships between fatigue and swing coordination patterns.
To improve ecological validity, future research should incorporate field-based experiments that more accurately simulate the complex demands of actual golf performance. Third, the current study’s fatigue protocols may not precisely represent the specific challenges of competitive golf. While the Stroop task served as our central fatigue induction method (a well-established cognitive test), it may not fully capture the mental demands of an actual golf game. Similarly, the plank-based peripheral fatigue protocol might not adequately reproduce the exact muscular fatigue patterns generated during golf swings. Future investigations should develop sport-specific fatigue protocols that better replicate golf’s unique physical and cognitive requirements.
Additionally, while movement variability served as our primary outcome measure, we acknowledge that increased variability does not necessarily indicate impaired performance. In certain contexts, greater variability may demonstrate beneficial motor adaptability and system resilience. Addressing these limitations in subsequent studies will advance our understanding of how central and peripheral fatigue affect coordination patterns in golf and other precision sports.
Conclusion
The findings of this study reveal that the motor coordination pattern variability in the muscular fatigue group was significantly greater than that of the control group but lower than that of the central fatigue group. Notably, the central fatigue group demonstrated the highest coordination variability, underscoring the profound influence of central fatigue on motor control. This research provides the first simultaneous investigation of both peripheral and central fatigue effects on golf swing mechanics, suggesting the need for additional studies to examine these relationships across different protocols and sporting contexts.
Based on these results, we recommend that coaches and instructors employ targeted strategies to address both central and peripheral fatigue. For mental fatigue reduction, interventions, such as meditation, mental imagery, and mindfulness training may prove beneficial. Concurrently, enhancing physical conditioning can raise the threshold for peripheral fatigue onset. By implementing comprehensive fatigue management programs that optimize motivation and recovery, practitioners can help athletes sustain peak performance while minimizing injury risk.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Alzahra University, Tehran, Iran (Code: SSRI.REC-2211-1962). All ethical principles were strictly followed in this study. Participants were fully informed about the research objectives and procedures. They received assurances regarding data confidentiality and retained the right to withdraw from the study at any time. Participants were also informed that study results would be made available to them upon request. Written informed consent was obtained from all participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge all the participants for their involvement in this study.
References
- Kriswanto ES, Setijono H, Mintarto E. The effect of cardiorespiratory fitness and fatigue level on learning ability of motor coordination. Cakrawala Pendidikan. 2019; 38(2):320-9. [DOI:10.21831/cp.v38i2.24565]
- Hunter SK, Pereira HM, Keenan KG. The aging neuromuscular system and motor performance. Journal of Applied Physiology. 2016; 121(4):982-95. [DOI:10.1152/japplphysiol.00475.2016] [PMID]
- Dupuis F, Sole G, Mercier C, Roy JS. Impact of fatigue at the shoulder on the contralateral upper limb kinematics and performance. Plos One. 2022; 17(4):e0266370. [DOI:10.1371/journal.pone.0266370] [PMID]
- Tornero-Aguilera JF, Jimenez-Morcillo J, Rubio-Zarapuz A, Clemente-Suárez VJ. Central and peripheral fatigue in physical exercise explained: A narrative review. International Journal of Environmental Research and Public Health. 2022; 19(7):3909. [DOI:10.3390/ijerph19073909] [PMID]
- Izadi A, TahmasebiBoroujeni S, Doosti M. Effect of central and peripheral fatigue on throwing accuracy and velocity in handball. Sport Psychology Studies. 2020; 9(31):245-62. [Link]
- Ortiz A, Olson SL, Etnyre B, Trudelle-Jackson EE, Bartlett W, Venegas-Rios HL. Fatigue effects on knee joint stability during two jump tasks in women. The Journal of Strength & Conditioning Research. 2010; 24(4):1019-27. [DOI:10.1519/JSC.0b013e3181c7c5d4] [PMID]
- Bernardo F, Martins R, Guedes JC. Evaluation of physical fatigue based on motion analysis. In: Arezes PM, Baptista JS, Barroso MP, Carneiro P, Costa N, Melo RB, Miguel S, Perestrelo G, editors. Occupational Safety and Hygiene VI. Boca Raton: CRC Press; 2018. [DOI:10.1201/9781351008884-13]
- Chen XX, Ji ZG, Wang Y, Xu J, Wang LY, Wang HB. Bibliometric analysis of the effects ofcentral fatigue on athletic performance from 2001 to 2021. Frontiers in Psychology. 2023; 13:1019417. [DOI:10.3389/fpsyg.2022.1019417] [PMID]
- Gantois P, Caputo Ferreira ME, Lima-Junior D, Nakamura FY, Batista GR, Fonseca FS, et al. Effects of mental fatigue on passing decision-making performance in professional soccer athletes. European Journal of Sport Science. 2020; 20(4):534-43. [DOI:10.1080/17461391.2019.1656781] [PMID]
- Smith MR, Zeuwts L, Lenoir M, Hens N, De Jong LM, Coutts AJ. Mental fatigue impairs soccer-specific decision-making skill. Journal of Sports Sciences. 2016; 34(14):1297-304. [DOI:10.1080/02640414.2016.1156241] [PMID]
- Hasan MS, Haidary M, Gandomi F. [The effect of eight weeks yoga training on the mental fatigue control and balance, lower extremity function and landing mechanic in physical education students (Persian)]. Journal for Research in Sport Rehabilitation. 2020; 7(14):57-69. [DOI:10.22084/rsr.2020.21620.1507]
- Lew FL, Qu X. Effects ofcentral fatigue on biomechanics of slips. Ergonomics. 2014; 57(12):1927-32. [DOI:10.1080/00140139.2014.937771] [PMID]
- Higdon NR, Finch WH, Leib D, Dugan EL. Effects of fatigue on golf performance. Sports Biomechanics. 2012; 11(2):190-6. [DOI:10.1080/14763141.2011.638386] [PMID]
- Hakukawa S, Harato K, Morita E, Nishizawa K, Kobayashi S, Niki Y, et al. Biomechanical correlation between trunk and foot kinematics during golf swing movement before and after fatigue. 2021 [Unpublished]. [DOI:10.21203/rs.3.rs-651875/v1]
- Ricardo D, Teles J, Raposo MR, Veloso AP, João F. Test-Retest Reliability of a 6DoF Marker Set for Gait Analysis in Cerebral Palsy Children. Applied Sciences. 2021; 11(14):6515. [DOI:10.3390/app11146515]
- Verschueren JO, Tassignon B, Proost M, Teugels A, VAN Cutsem J, Roelands B, et al. Does Mental Fatigue Negatively Affect Outcomes of Functional Performance Tests? Medicine and Science in Sports and Exercise. 2020; 52(9):2002-10. [DOI:10.1249/MSS.0000000000002323] [PMID]
- Gebel A, Busch A, Stelzel C, Hortobágyi T, Granacher U. Effects of physical andcentral fatigue on postural sway and cortical activity in healthy young adults. Frontiers in Human Neuroscience. 2022; 16:871930. [DOI:10.3389/fnhum.2022.871930] [PMID]
- Williams N. The Borg rating of perceived exertion (RPE) scale. Occupational Medicine. 2017; 67(5):404-5. [DOI:10.1093/occmed/kqx063]
- Srinivasan AG, Smith SS, Pattinson CL, Mann D, Sullivan K, Salmon P, et al. Heart rate variability as an indicator of fatigue: A structural equation model approach. Transportation Research Part F: Traffic Psychology and Behaviour. 2024; 103:420-9. [DOI:10.1016/j.trf.2024.04.015]
- Zahiri A, Shahbazi M, Kordi MR, Fazel Kalkhoran J. [The effect of central and peripheral fatigue on coordination of student athletes (Persian)]. Journal of Sports and Motor Development and Learning. 2017; 9(1):123-36. [DOI:10.22059/jmlm.2017.61951]
- Smith MR, Chai R, Nguyen HT, Marcora SM, Coutts AJ. Comparing the effects of three cognitive tasks on indicators of mental fatigue. The Journal of Psychology. 2019; 153(8):759-83. [DOI:10.1080/00223980.2019.1611530] [PMID]
- Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in humans. Journal of Applied Physiology. 2009; 106(3):857-64. [DOI:10.1152/japplphysiol.91324.2008] [PMID]
- Lederman E. Neuromuscular rehabilitation in manual and physical therapy. Edinburgh: Churchill Livingstone; 2010. [Link]
- Martin K, Meeusen R, Thompson KG, Keegan R, Rattray B. Mental fatigue impairs endurance performance: A physiological explanation. Sports Medicine. 2018; 48(9):2041-51. [DOI:10.1007/s40279-018-0946-9] [PMID]
- Rafiee Z, Mikaili F. [The effect of cognitive exhaustion on cognitive flexibility with regard mediating need for cognition in Arak University teacher in the year 2015-16 (Persian)]. Cognitive Psychology Journal. 2019; 7(1):43-60. [Link]
- Skala F, Zemkova E. Effects of acute fatigue on cognitive performance in team sport players: Does it change the way they perform? A scoping review. Applied Sciences. 2022; 12(3):1736. [DOI:10.3390/app12031736]
- De Vleeschouwer E. The influence of mental fatigue on lower limb landing kinematics [PhD dissertation]. Ghent: Ghent University; 2021. [Link]
- Langner R, Steinborn MB, Chatterjee A, Sturm W, Willmes K. Mental fatigue and temporal preparation in simple reaction-time performance. Acta Psychologica. 2010; 133(1):64-72. [DOI:10.1016/j.actpsy.2009.10.001] [PMID]
- Pajoutan M, Ghesmaty Sangachin M, Cavuoto LA. Central and peripheral fatigue development in the shoulder muscle with obesity during an isometric endurance task. BMC Musculoskeletal Disorders. 2017; 18(1):314. [DOI:10.1186/s12891-017-1676-0] [PMID]
- Cowley JC, Gates DH. Inter-joint coordination changes during and after muscle fatigue. Human Movement Science. 2017; 56(Pt B):109-18. [DOI:10.1016/j.humov.2017.10.015] [PMID]
- Uygur M, Goktepe A, Ak E, Karabörk H, Korkusuz F. The effect of fatigue on the kinematics of free throw shooting in basketball. Journal of Human Kinetics. 2010; 24(2010):51-6. [DOI:10.2478/v10078-010-0019-0]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. The Journal of Physiology. 2008; 586(1):11-23. [DOI:10.1113/jphysiol.2007.139477] [PMID]
- Hunt MA, Hatfield GL. Ankle and knee biomechanics during normal walking following ankle plantarflexor fatigue. Journal of Electromyography and Kinesiology. 2017; 35:24-9. [DOI:10.1016/j.jelekin.2017.05.007] [PMID]
- Afhami N, Sahebozamani M, Mohamadi Por F. [Comparison of the Effects of Muscular Fatigue on Neck Proprioception Performance between Professional Karate Athletes and Non-Athletes (Persian)]. The Scientific Journal of Rehabilitation Medicine. 2017; 6(3):1-10. [DOI:10.22037/jrm.2017.1100332]
- Gao Z, Fekete G, Baker JS, Liang M, Xuan R, Gu Y. Effects of running fatigue on lower extremity symmetry among amateur runners: From a biomechanical perspective. Frontiers in Physiology. 2022; 13:899818. [DOI:10.3389/fphys.2022.899818] [PMID]
- Chaharmahali L, Rafei M, Azadian E. Effects of shoulder complex muscles fatigue in hand’s fine and gross skills in volleyball players. The Scientific Journal of Rehabilitation Medicine. 2019; 8(4):39-46. [DOI:10.22037/jrm.2019.111500.2035]
- Lavender AP, Balkozak S, Özyurt MG, Topkara B, Karacan İ, Bilici İ, et al. Effect of aging on H-reflex response to fatigue. Experimental Brain Research. 2020; 238(2):273-82. [DOI:10.1007/s00221-019-05708-7] [PMID]
- Van Cutsem J, Marcora S, De Pauw K, Bailey S, Meeusen R, Roelands B. The effects of mental fatigue on physical performance: A systematic review. Sports Medicine. 2017; 47(8):1569-88. [DOI:10.1007/s40279-016-0672-0] [PMID]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nature Neuroscience. 2002; 5(11):1226-35. [DOI:10.1038/nn963] [PMID]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998; 394(6695):780-4. [DOI:10.1038/29528]
- Srinivasan D, Mathiassen SE. Motor variability in occupational health and performance. Clinical Biomechanics. 2012; 27(10):979-93. [DOI:10.1016/j.clinbiomech.2012.08.007] [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/06/29 | Accepted: 2025/05/31 | Published: 2026/01/1
Received: 2024/06/29 | Accepted: 2025/05/31 | Published: 2026/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






