Wed, Dec 10, 2025
Volume 13, Issue 4 (Autumn 2023)
PTJ 2023, 13(4): 215-224 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fatollahi A, Jafarnezhadgero A A, Sheikhalizade H. The Effect of Sand Exercise Program on Knee Muscle Co-contraction in Runners With Over-pronated Feet During Running. PTJ 2023; 13 (4) :215-224
URL: http://ptj.uswr.ac.ir/article-1-582-en.html
URL: http://ptj.uswr.ac.ir/article-1-582-en.html
1- Department of Sport Biomechanics, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran.
Full-Text [PDF 519 kb]
(911 Downloads)
| Abstract (HTML) (2793 Views)
Full-Text: (543 Views)
1. Introduction
Running is the most popular form of recreational physical activity in the world [1]. Running-related injuries may be associated with the type of sport and lower limb abnormality [2]. Over the past decades, over-pronated foot (OPF) has been discussed as a potential risk factor for injuries or as the mechanism behind impact damping [3].
People with OPF demonstrate pain in the knee joint [4]. OPF deformity can lead to lower extremity dysfunction [5]. OPF alters lower extremity mechanics during daily activities [6]. Also, OPF is recognized as a running-related injury risk factor [5]. However, it is not clear whether the knee muscles are activated synchronously to modulate joint functions [7, 8].
The association between the agonist/antagonist muscular activations is assessed using surface electromyography [9]. Muscular co-contraction is the contraction of agonist and antagonist muscles at a joint simultaneously. Muscle co-contraction could be regarded as a main factor during training protocols [10, 11]. The increased levels of medial muscle co-contraction and lateral muscle co-contraction were reported in the osteoarthritis patients [12].
Training on sand produced higher muscle activities of the lower limbs with lower ground reaction forces during running and walking in individuals with OPF [13, 14]. An exercise program on an unstable surface, such as sand could be useful [15]. As such, a greater understanding of the sand exercise program is necessary to fully gauge the extent of its application to training methods in individuals with OPF. There is limited investigation on the effects of a sand exercise program on knee muscular co-contraction in OPF individuals.
2. Materials and Methods
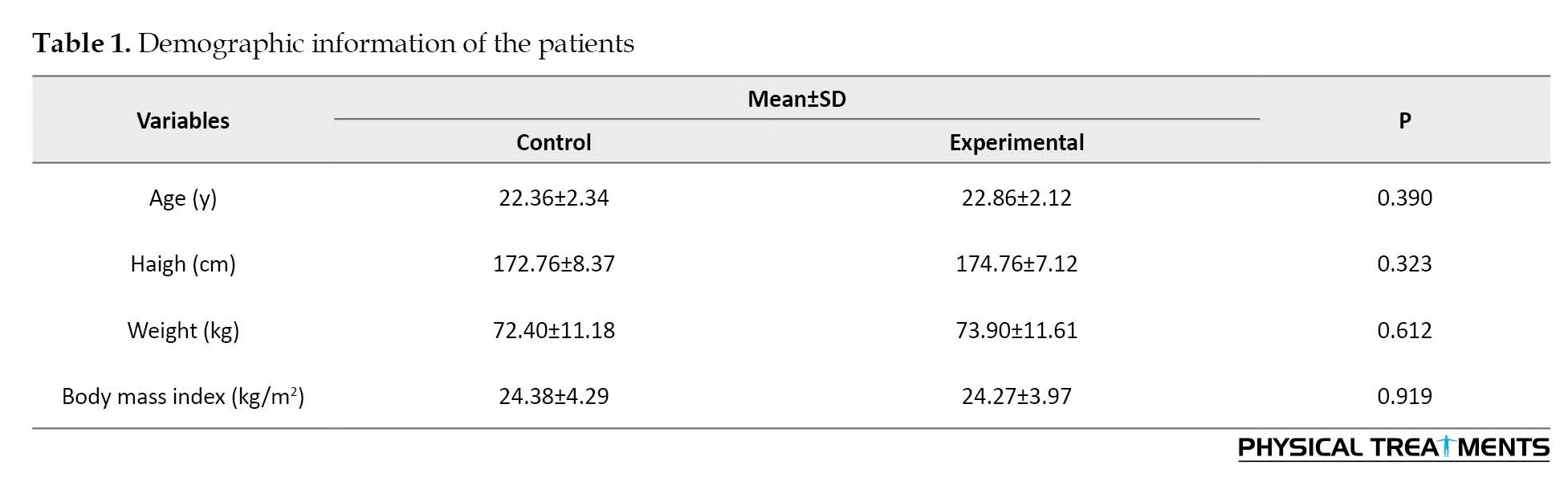
The study type was a randomized controlled trial. The G*Power software, version 3.1 for the F-test family (repeated-measures ANOVA for within-between interaction) was used for a priori power analysis (type I error=0.05, type II error rate=0.20 (80% statistical power), and effect size=0.80). The G*Power software showed that at least 30 participants would be sufficient. Participants (age range: 18–26 years) were recruited from local clinics in Ardabil City. Thirty runners with OPF were considered for the control group and 30 runners with OPF for the experimental group (Table 1).
For both groups, participants were recruited if they showed a navicular drop >10 mm [16] and a foot posture index >10 [16]. Exclusion criteria included musculoskeletal surgery history, orthopedic disorders (except for OPF), and limb length differences >5 mm.
An electromyography (EMG) system (Biometrics Ltd, Newport, UK) with Ag/AgCl electrodes were used to record the activity of the medial gastrocnemius (Gas-M), biceps femoris (BF), semitendinosus (ST), vastus lateralis (VL), vastus medialis (VM), and rectus femoris (RF) muscles of the right limb [17]. The raw EMG signals were sampled at 1000 Hz. According to the SENIAM protocol, the skin surface of the selected muscles was cleaned and shaved with alcohol. The running was divided into the loading (0%-20% gait cycle), mid-stance (20%-47% gait cycle), push-off (47%-70% gait cycle), and swing (70%-100% gait cycle) phases. Maximum voluntary isometric contraction (MVIC) was used for the normalization process of EMG data during running.
Two types of co-contraction were calculated as follows: 1) Directed co-contraction (DCC) and 2) General co-contraction (GCC). DCC ratios were used for the medial (ST, VM, Gas-M)/lateral (BF, VL) direction (DCCML), medial (ST)/lateral (BF) hamstrings (DCCMLH), medial (VM)/lateral (VL) quadriceps (DCCMLQ), and the knee flexors (ST, BF, Gas-M)/extensors (VL, VM, RF) (DCCFE). The DCC ratios were assessed as Equation 1 [18]:
1. If agonist amplitude > antagonist amplitude;
DCC=1-Antagonist amplitude/Agonist amplitude
DCC=Agonist amplitude/Antagonist amplitude-1
Maximum directed co-contraction would be equal to zero and minimum directed co-contraction would be numbers approached to ratio 1 or -1 [18]. For GCC values, muscular activities were calculated from the normalized EMG data during each stance sub-phase and swing phase of running.
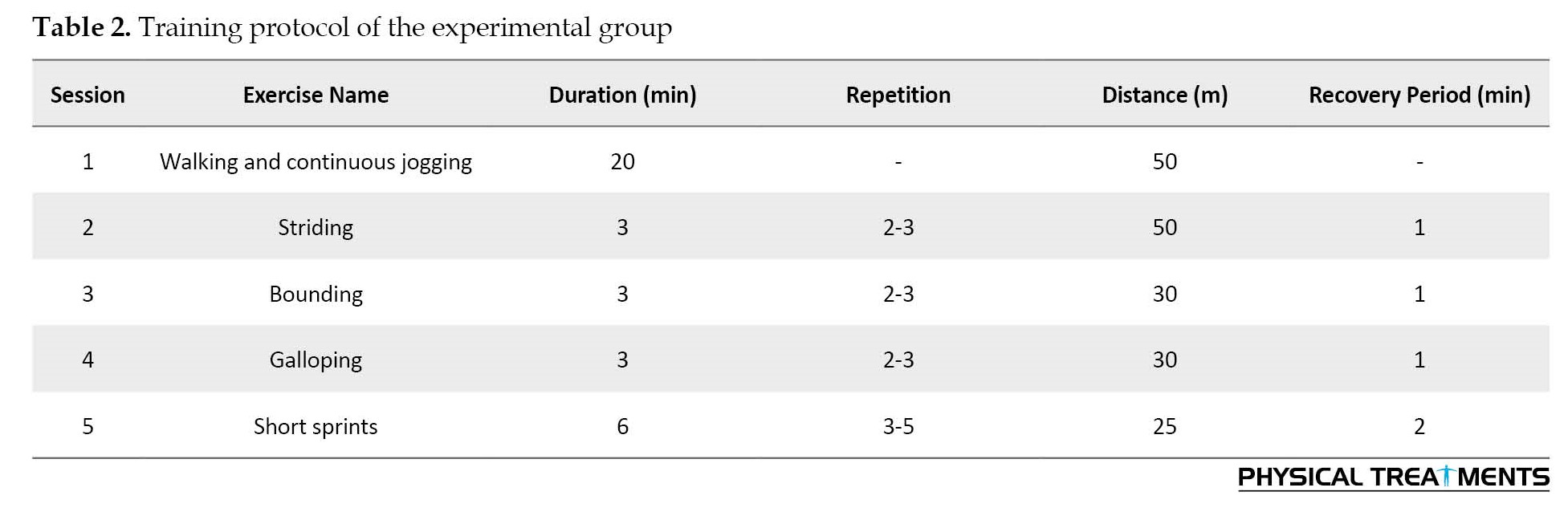
The intervention group performed training on sand consisting of walking and continuous running tasks for eight weeks (three sessions per week) [19]. Every session was done with a warm-up and stretching session for 5 minutes and ended with a cool-down session for 5 minutes [19]. The total training period was 50 minutes per session [19] (Table 2).
Participants conducted a 5-minute warm-up protocol. For the running trials, participants were familiarized with the laboratory situation and ran across runway three times. After the running trials, MVIC exercises were performed for each muscle. Five successful running trials were recorded and used for further data analysis. This process was performed for the groups in two steps “pre-test and post-test”.
The normal distribution of data was examined and confirmed using the Shapiro-Wilk test, and an independent samples t-test was used to determine baseline between-group differences. For this purpose, the repeated-measures two-way ANOVA was used. Post hoc analyses were done using the Bonferroni test and paired sample t-test. The significance level was 0.05. All analyses were done using SPSS software, version 20.0.
3. Results
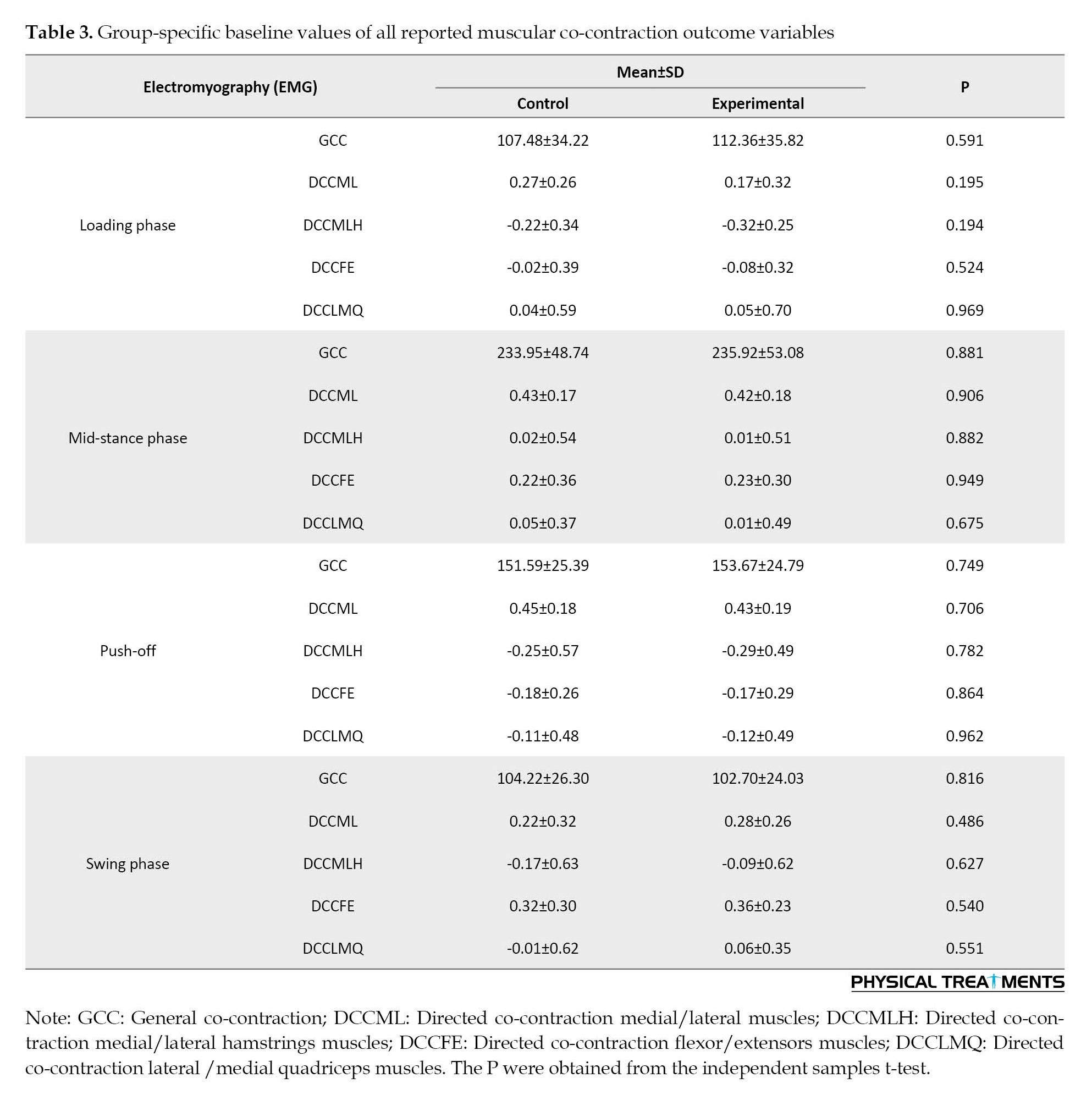
Participants’ outcome variables at baseline are illustrated in Table 3.
The results did not show the significant effect of “time”, “group”, and group×time interaction for knee joint co-contraction in the loading phase (P>0.05) (Table 4).
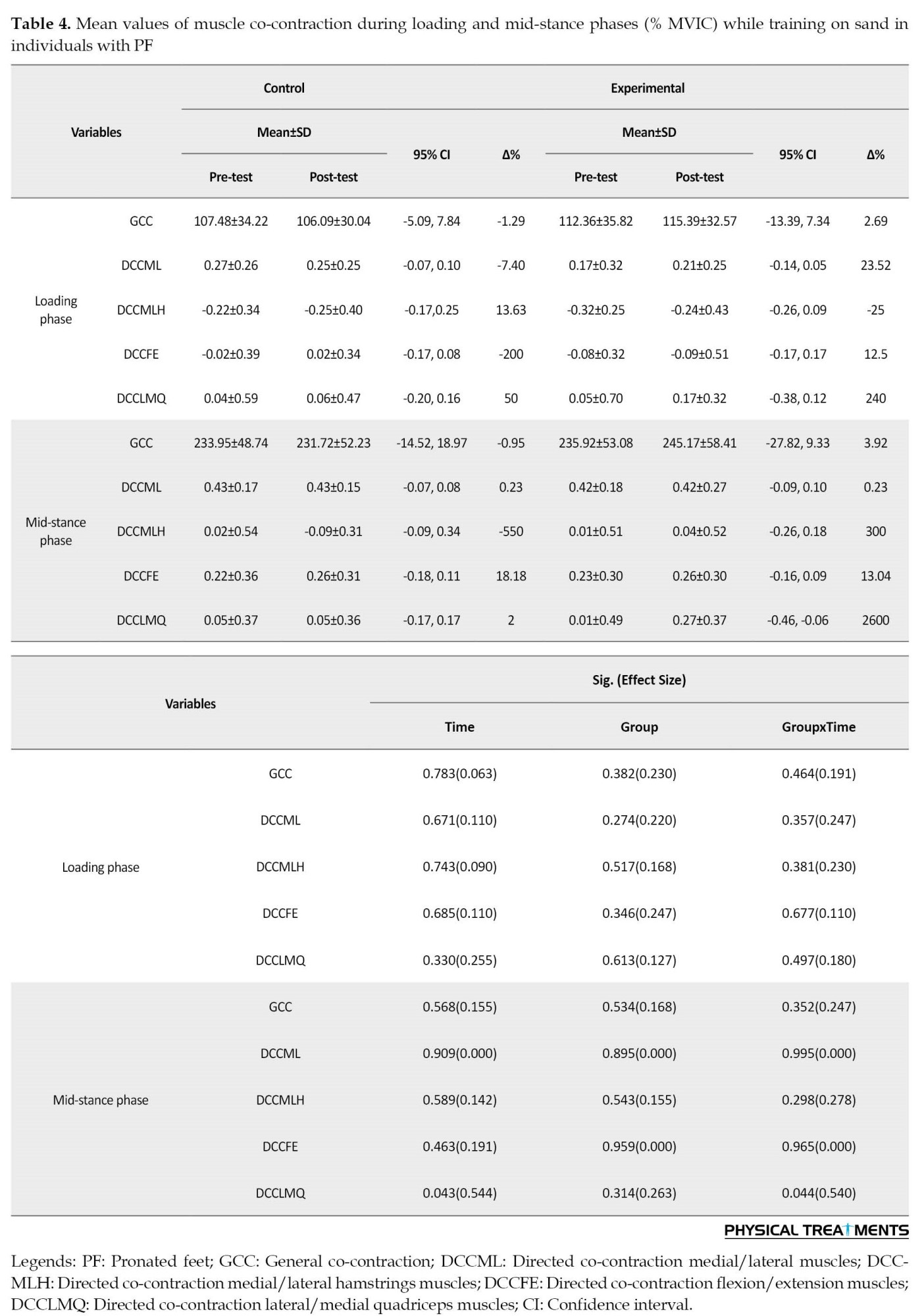
Significant effects of “time” were found for directed lateral/medial knee co-contraction during the mid-stance phase (P<0.043; d=0.544) (Table 4). The results revealed significantly lower directed lateral/medial knee co-contraction (P=0.043; d=0.602) in the post-test than the pre-test (Table 4). Group×time interactions were significant for directed lateral/medial knee co-contraction at the mid-stance (P<0.044; d=0.540) (Table 4). In the intervention group, significantly lower directed lateral/medial knee co-contraction (P=0.044, d=651) was found in the post-test compared to the pre-test (Table 4).
Of note, significant effects of “time” were observed for general and medial/lateral knee co-contraction in the push-off phase (P<0.033; d=0.574-0.648) (Table 5).
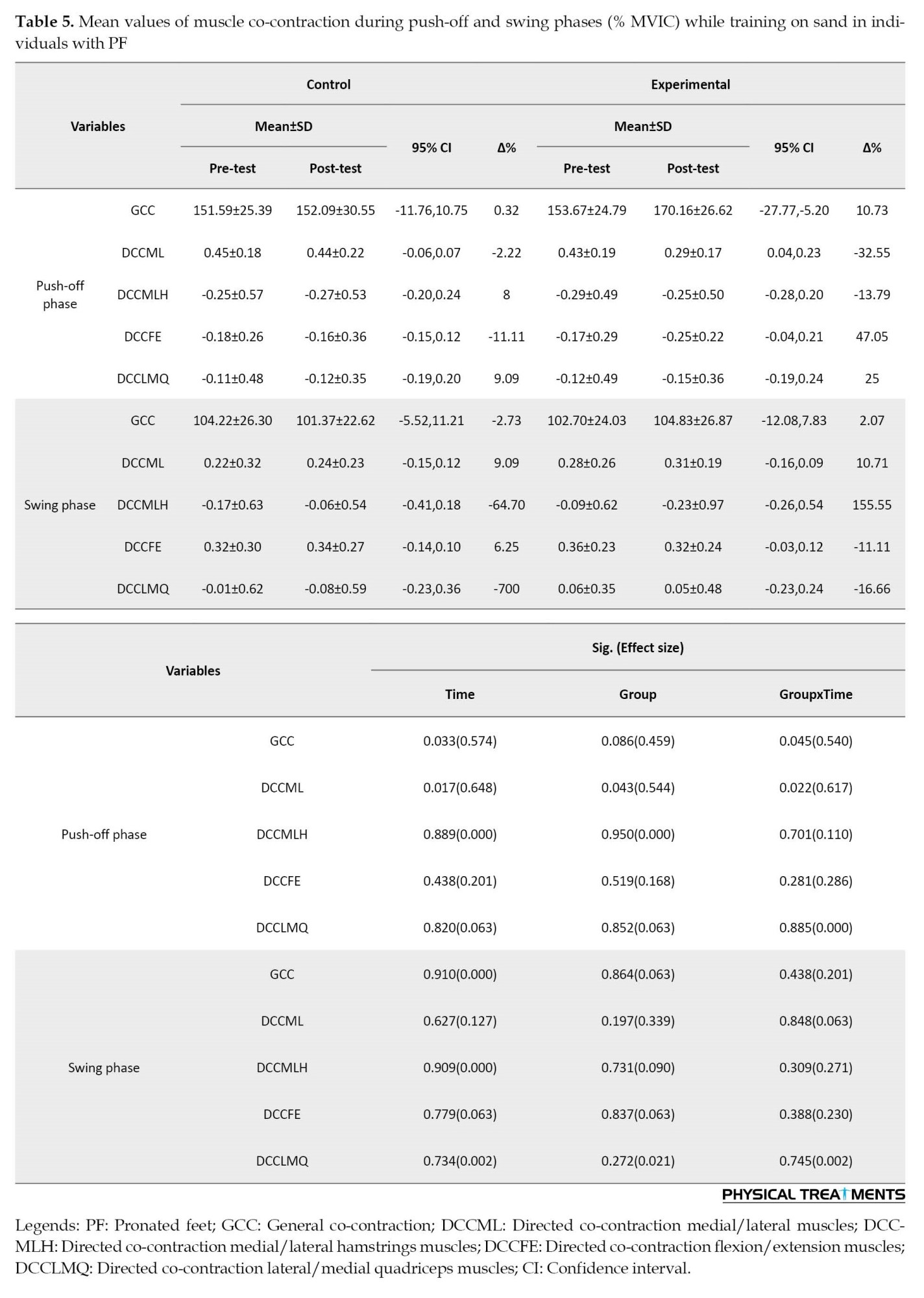
Pair-wise comparisons revealed significantly greater general (P=0.033; d=0.445) and directed medial/lateral co-contraction of the knee muscles (P=0.017; d=0.506) in the post-test (Table 5).
Also, the findings demonstrated significant main effects of “group” for directed medial/lateral co-contraction of the knee muscles in the push-off phase (P<0.043; d=0.544) (Table 5). The results showed significantly greater directed medial/lateral co-contraction (P=0.043; d=0.527) of knee muscles in the experimental group compared to the control group (Table 5).
Finally, we found the significant effect of group×time interaction for general co-contraction and directed medial/lateral co-contraction of knee muscles during the push-off phase (P<0.045; d=0.540-617) (Table 5). In the experimental group but not the control group, significantly greater general co-contraction (P=0.045, d=611) and directed medial/lateral co-contraction (P=0.022, d=707) of knee muscles were found in the post-test compared to the pre-test (Table 5).
No statistically significant main effects of “time” were found for the co-contraction of knee muscles during the swing phase (P>0.05; d=0.000-0.127) (Table 5). Also, the statistical analyses did not demonstrate any significant main effects of “group” for co-contraction of knee muscles during the swing phase (P>0.05; d=0.021-0.339) (Table 5). Finally, we found no significant effect of group×time interaction for co-contraction of knee muscles during the swing phase (P>0.05; d=0.002-0.271) (Table 5).
4. Discussion
This study aimed to evaluate the effect of a sand exercise program on knee muscle co-contraction in runners with OPF. Main results included: I) Lower directed co-contraction lateral/medial quadriceps of knee muscles during the mid-stance phase were observed in the intervention group at the post-test; significantly greater general co-contraction and directed medial/lateral co-contraction of knee muscles were observed in the intervention group at the post-test during push-off phase; II) Irrespective of the group, greater general co-contraction and directed medial/lateral co-contraction of knee muscles during the push-off phase were observed at the post-test; III) Irrespective of the time, greater directed medial/lateral co-contraction of knee muscles during the push-off phase were observed in the intervention group during sand exercise program.
Co-contraction values could be associated with internal force values and may be important for injury prevention. The neuro-muscular system adjusts mechanical joint stiffness through muscle activation level [20]. Moreover, reduced range of motion has also been associated with reduced limb stability [20]. The individuals with OPF demonstrated poor knee joint stability following external perturbation [21]. Underpinning the pathomechanisms of knee joint instability associated with OPF structural alignment may provide a better understanding of the prevention and treatment of knee joint injuries [21]. The regulatory role of the co-contraction between flexor and extensor muscles provides knee joint stability [22]. Higher ankle co-contraction is associated with better ankle stability but could increase quadriceps to hamstrings co-contraction [23]. Greater general and directed medial/lateral co-contraction of knee muscles were found at the post-test during push-off. Relevant training programs may help runners with OPF to reduce atypical knee loading during the loading phase and improve knee stability.
Reduction of range of motion has been linked to an increase in ankle and knee hardness [24, 25]. It has been shown that lower vertical leg motion resulted in an increase in stiffness on softer surfaces [26]. Although the sand exercise program was used in this study, it may produce some instability, which is controlled through increasing stiffness levels [27]. The individuals with OPF demonstrated poor knee joint stability following external perturbation [21]. Underpinning the pathomechanisms of knee joint instability associated with OPF structural alignment may provide a better understanding of anterior cruciate ligament (ACL) injuries and a theoretical basis for more accurate clinical diagnosis, prevention, and treatment of knee joint injuries [21]. In the training group, both the general co-contraction and directed medial/lateral co-contraction of knee muscles were significantly greater in the post-test during the push-off phase. Relevant training programs may help runners with OPF to reduce atypical knee loading during the loading phase and improve knee stability.
Higher medial-lateral knee muscular co-contraction could lead to knee osteoarthritis [28]. Muscular activities could increase or decrease the rate of injury occurrences [28]. Previous studies have provided conflicting findings regarding muscular activity levels in OA patients [29, 30]. For example, it has been reported that lateral muscle co-activity in moderate OA is greater than in healthy ones [31]. The increased co-contraction of agonist and antagonist muscles may be interpreted as an attempt to increase lower-limb joint stability [32]. Muscle co-contraction around the knee joint is an important part of normal neuromuscular control [33]. Co-contraction could support joint ligaments in order to maintain joint stability and be used for injury prevention [33]. Training programs may have positive effects for individuals with PF to reduce knee injuries during running by the greater directed medial/lateral co-contraction of knee muscles.
Limitations in the current study are acknowledged. The first limitation of the present data is the lack of temporal muscle activation values. Second, we did not record kinematic data in this study. Third, we only tested males, and therefore, our results may not be applicable to females.
5. Conclusions
This study identified co-contraction organizations of the knee muscles that contribute to stability in the OPF during running. Runners with OPF demonstrated poor knee joint stability. We observed greater co-contraction of knee muscles during a sand exercise program. Therefore, training on sand may help runners with OPF improve knee stability and reduce atypical knee loading and injuries while running.
Ethical Considerations
Compliance with ethical guidelines
Informed consent was obtained from the participants in accordance with the Declaration of Helsinki. The right foot was the dominant limb for all participants. This study was approved by the local Ethics Committee of Ardabil University of Medical Sciences (Code: IR.ARUMS.REC.1398.484).
Funding
This paper was registered at the Iranian Registry of Clinical Trial (IRCT) (Code: IRCT20191211045704N1).
Authors' contributions
Conceptualization and methodology: Amir Fatollahi and Amir Ali Jafarnezhadgero; Data collection, data analysis and original draft preparation: Amir Fatollahi and Hamed Sheikhalizade; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
The authors gratefully thank all participants for participating in this study.
References
Running is the most popular form of recreational physical activity in the world [1]. Running-related injuries may be associated with the type of sport and lower limb abnormality [2]. Over the past decades, over-pronated foot (OPF) has been discussed as a potential risk factor for injuries or as the mechanism behind impact damping [3].
People with OPF demonstrate pain in the knee joint [4]. OPF deformity can lead to lower extremity dysfunction [5]. OPF alters lower extremity mechanics during daily activities [6]. Also, OPF is recognized as a running-related injury risk factor [5]. However, it is not clear whether the knee muscles are activated synchronously to modulate joint functions [7, 8].
The association between the agonist/antagonist muscular activations is assessed using surface electromyography [9]. Muscular co-contraction is the contraction of agonist and antagonist muscles at a joint simultaneously. Muscle co-contraction could be regarded as a main factor during training protocols [10, 11]. The increased levels of medial muscle co-contraction and lateral muscle co-contraction were reported in the osteoarthritis patients [12].
Training on sand produced higher muscle activities of the lower limbs with lower ground reaction forces during running and walking in individuals with OPF [13, 14]. An exercise program on an unstable surface, such as sand could be useful [15]. As such, a greater understanding of the sand exercise program is necessary to fully gauge the extent of its application to training methods in individuals with OPF. There is limited investigation on the effects of a sand exercise program on knee muscular co-contraction in OPF individuals.
2. Materials and Methods
The study type was a randomized controlled trial. The G*Power software, version 3.1 for the F-test family (repeated-measures ANOVA for within-between interaction) was used for a priori power analysis (type I error=0.05, type II error rate=0.20 (80% statistical power), and effect size=0.80). The G*Power software showed that at least 30 participants would be sufficient. Participants (age range: 18–26 years) were recruited from local clinics in Ardabil City. Thirty runners with OPF were considered for the control group and 30 runners with OPF for the experimental group (Table 1).

For both groups, participants were recruited if they showed a navicular drop >10 mm [16] and a foot posture index >10 [16]. Exclusion criteria included musculoskeletal surgery history, orthopedic disorders (except for OPF), and limb length differences >5 mm.
An electromyography (EMG) system (Biometrics Ltd, Newport, UK) with Ag/AgCl electrodes were used to record the activity of the medial gastrocnemius (Gas-M), biceps femoris (BF), semitendinosus (ST), vastus lateralis (VL), vastus medialis (VM), and rectus femoris (RF) muscles of the right limb [17]. The raw EMG signals were sampled at 1000 Hz. According to the SENIAM protocol, the skin surface of the selected muscles was cleaned and shaved with alcohol. The running was divided into the loading (0%-20% gait cycle), mid-stance (20%-47% gait cycle), push-off (47%-70% gait cycle), and swing (70%-100% gait cycle) phases. Maximum voluntary isometric contraction (MVIC) was used for the normalization process of EMG data during running.
Two types of co-contraction were calculated as follows: 1) Directed co-contraction (DCC) and 2) General co-contraction (GCC). DCC ratios were used for the medial (ST, VM, Gas-M)/lateral (BF, VL) direction (DCCML), medial (ST)/lateral (BF) hamstrings (DCCMLH), medial (VM)/lateral (VL) quadriceps (DCCMLQ), and the knee flexors (ST, BF, Gas-M)/extensors (VL, VM, RF) (DCCFE). The DCC ratios were assessed as Equation 1 [18]:
1. If agonist amplitude > antagonist amplitude;
DCC=1-Antagonist amplitude/Agonist amplitude
DCC=Agonist amplitude/Antagonist amplitude-1
Maximum directed co-contraction would be equal to zero and minimum directed co-contraction would be numbers approached to ratio 1 or -1 [18]. For GCC values, muscular activities were calculated from the normalized EMG data during each stance sub-phase and swing phase of running.
The intervention group performed training on sand consisting of walking and continuous running tasks for eight weeks (three sessions per week) [19]. Every session was done with a warm-up and stretching session for 5 minutes and ended with a cool-down session for 5 minutes [19]. The total training period was 50 minutes per session [19] (Table 2).

Participants conducted a 5-minute warm-up protocol. For the running trials, participants were familiarized with the laboratory situation and ran across runway three times. After the running trials, MVIC exercises were performed for each muscle. Five successful running trials were recorded and used for further data analysis. This process was performed for the groups in two steps “pre-test and post-test”.
The normal distribution of data was examined and confirmed using the Shapiro-Wilk test, and an independent samples t-test was used to determine baseline between-group differences. For this purpose, the repeated-measures two-way ANOVA was used. Post hoc analyses were done using the Bonferroni test and paired sample t-test. The significance level was 0.05. All analyses were done using SPSS software, version 20.0.
3. Results
Participants’ outcome variables at baseline are illustrated in Table 3.

The results did not show the significant effect of “time”, “group”, and group×time interaction for knee joint co-contraction in the loading phase (P>0.05) (Table 4).

Significant effects of “time” were found for directed lateral/medial knee co-contraction during the mid-stance phase (P<0.043; d=0.544) (Table 4). The results revealed significantly lower directed lateral/medial knee co-contraction (P=0.043; d=0.602) in the post-test than the pre-test (Table 4). Group×time interactions were significant for directed lateral/medial knee co-contraction at the mid-stance (P<0.044; d=0.540) (Table 4). In the intervention group, significantly lower directed lateral/medial knee co-contraction (P=0.044, d=651) was found in the post-test compared to the pre-test (Table 4).
Of note, significant effects of “time” were observed for general and medial/lateral knee co-contraction in the push-off phase (P<0.033; d=0.574-0.648) (Table 5).

Pair-wise comparisons revealed significantly greater general (P=0.033; d=0.445) and directed medial/lateral co-contraction of the knee muscles (P=0.017; d=0.506) in the post-test (Table 5).
Also, the findings demonstrated significant main effects of “group” for directed medial/lateral co-contraction of the knee muscles in the push-off phase (P<0.043; d=0.544) (Table 5). The results showed significantly greater directed medial/lateral co-contraction (P=0.043; d=0.527) of knee muscles in the experimental group compared to the control group (Table 5).
Finally, we found the significant effect of group×time interaction for general co-contraction and directed medial/lateral co-contraction of knee muscles during the push-off phase (P<0.045; d=0.540-617) (Table 5). In the experimental group but not the control group, significantly greater general co-contraction (P=0.045, d=611) and directed medial/lateral co-contraction (P=0.022, d=707) of knee muscles were found in the post-test compared to the pre-test (Table 5).
No statistically significant main effects of “time” were found for the co-contraction of knee muscles during the swing phase (P>0.05; d=0.000-0.127) (Table 5). Also, the statistical analyses did not demonstrate any significant main effects of “group” for co-contraction of knee muscles during the swing phase (P>0.05; d=0.021-0.339) (Table 5). Finally, we found no significant effect of group×time interaction for co-contraction of knee muscles during the swing phase (P>0.05; d=0.002-0.271) (Table 5).
4. Discussion
This study aimed to evaluate the effect of a sand exercise program on knee muscle co-contraction in runners with OPF. Main results included: I) Lower directed co-contraction lateral/medial quadriceps of knee muscles during the mid-stance phase were observed in the intervention group at the post-test; significantly greater general co-contraction and directed medial/lateral co-contraction of knee muscles were observed in the intervention group at the post-test during push-off phase; II) Irrespective of the group, greater general co-contraction and directed medial/lateral co-contraction of knee muscles during the push-off phase were observed at the post-test; III) Irrespective of the time, greater directed medial/lateral co-contraction of knee muscles during the push-off phase were observed in the intervention group during sand exercise program.
Co-contraction values could be associated with internal force values and may be important for injury prevention. The neuro-muscular system adjusts mechanical joint stiffness through muscle activation level [20]. Moreover, reduced range of motion has also been associated with reduced limb stability [20]. The individuals with OPF demonstrated poor knee joint stability following external perturbation [21]. Underpinning the pathomechanisms of knee joint instability associated with OPF structural alignment may provide a better understanding of the prevention and treatment of knee joint injuries [21]. The regulatory role of the co-contraction between flexor and extensor muscles provides knee joint stability [22]. Higher ankle co-contraction is associated with better ankle stability but could increase quadriceps to hamstrings co-contraction [23]. Greater general and directed medial/lateral co-contraction of knee muscles were found at the post-test during push-off. Relevant training programs may help runners with OPF to reduce atypical knee loading during the loading phase and improve knee stability.
Reduction of range of motion has been linked to an increase in ankle and knee hardness [24, 25]. It has been shown that lower vertical leg motion resulted in an increase in stiffness on softer surfaces [26]. Although the sand exercise program was used in this study, it may produce some instability, which is controlled through increasing stiffness levels [27]. The individuals with OPF demonstrated poor knee joint stability following external perturbation [21]. Underpinning the pathomechanisms of knee joint instability associated with OPF structural alignment may provide a better understanding of anterior cruciate ligament (ACL) injuries and a theoretical basis for more accurate clinical diagnosis, prevention, and treatment of knee joint injuries [21]. In the training group, both the general co-contraction and directed medial/lateral co-contraction of knee muscles were significantly greater in the post-test during the push-off phase. Relevant training programs may help runners with OPF to reduce atypical knee loading during the loading phase and improve knee stability.
Higher medial-lateral knee muscular co-contraction could lead to knee osteoarthritis [28]. Muscular activities could increase or decrease the rate of injury occurrences [28]. Previous studies have provided conflicting findings regarding muscular activity levels in OA patients [29, 30]. For example, it has been reported that lateral muscle co-activity in moderate OA is greater than in healthy ones [31]. The increased co-contraction of agonist and antagonist muscles may be interpreted as an attempt to increase lower-limb joint stability [32]. Muscle co-contraction around the knee joint is an important part of normal neuromuscular control [33]. Co-contraction could support joint ligaments in order to maintain joint stability and be used for injury prevention [33]. Training programs may have positive effects for individuals with PF to reduce knee injuries during running by the greater directed medial/lateral co-contraction of knee muscles.
Limitations in the current study are acknowledged. The first limitation of the present data is the lack of temporal muscle activation values. Second, we did not record kinematic data in this study. Third, we only tested males, and therefore, our results may not be applicable to females.
5. Conclusions
This study identified co-contraction organizations of the knee muscles that contribute to stability in the OPF during running. Runners with OPF demonstrated poor knee joint stability. We observed greater co-contraction of knee muscles during a sand exercise program. Therefore, training on sand may help runners with OPF improve knee stability and reduce atypical knee loading and injuries while running.
Ethical Considerations
Compliance with ethical guidelines
Informed consent was obtained from the participants in accordance with the Declaration of Helsinki. The right foot was the dominant limb for all participants. This study was approved by the local Ethics Committee of Ardabil University of Medical Sciences (Code: IR.ARUMS.REC.1398.484).
Funding
This paper was registered at the Iranian Registry of Clinical Trial (IRCT) (Code: IRCT20191211045704N1).
Authors' contributions
Conceptualization and methodology: Amir Fatollahi and Amir Ali Jafarnezhadgero; Data collection, data analysis and original draft preparation: Amir Fatollahi and Hamed Sheikhalizade; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
The authors gratefully thank all participants for participating in this study.
References
- Johnson CD, Tenforde AS, Outerleys J, Reilly J, Davis IS. Impact-related ground reaction forces are more strongly associated with some running injuries than others. The American Journal of Sports Medicine. 2020 ; 48(12):3072-80.[DOI:10.1177/0363546520950731] [PMID]
- Francis P, Whatman C, Sheerin K, Hume P, Johnson MI. The proportion of lower limb running injuries by gender, anatomical location and specific pathology: A systematic review. Journal of Sports Science & Medicine. 2019; 18(1):21-31. [PMID] [PMCID]
- Zhang X, Vanwanseele B. Immediate effects of forefoot wedges on multi-segment foot kinematics during jogging in recreational runners with a symptomatic pronated foot. Frontiers in Physiology. 2023; 13:1064240. [DOI:10.3389/fphys.2022.1064240] [PMID]
- Farahpour N, Sharifmoradi K, Azizi S. Effect of fatigue on knee kinematics and kinetics during walking in individuals with flat feet. Physical Treatments-Specific Physical Therapy Journal. 2017; 7(3):141-8. [DOI:10.32598/ptj.7.3.141]
- Sung PS. Relative index of ankle muscle activations on agonistic phase between subjects with and without flat foot. Journal of Biomedical Engineering and Informatics. 2015; 2(1):129. [DOI:10.5430/jbei.v2n1p129]
- Kohls-Gatzoulis J, Angel JC, Singh D, Haddad F, Livingstone J, Berry G. Tibialis posterior dysfunction: a common and treatable cause of adult acquired flatfoot. BMJ. 2004; 329(7478):1328-33. [DOI:10.1136/bmj.329.7478.1328] [PMID]
- Farris DJ, Raiteri BJ. Modulation of leg joint function to produce emulated acceleration during walking and running in humans. Royal Society Open Science. 2017; 4(3):160901. [DOI:10.1098/rsos.160901] [PMID]
- Henriksen M, Alkjaer T, Lund H, Simonsen EB, Graven-Nielsen T, Danneskiold-Samsøe B, et al. Experimental quadriceps muscle pain impairs knee joint control during walking. Journal of Applied Physiology. 2007; 103(1):132-9.[DOI:10.1152/japplphysiol.01105.2006] [PMID]
- Teixeira da Fonseca S, Silva PL, Ocarino JM, Guimaràes RB, Oliveira MT, Lage CA. Analyses of dynamic co-contraction level in individuals with anterior cruciate ligament injury. Journal of Electromyography and Kinesiology. 2004; 14(2):239-47. [DOI:10.1016/j.jelekin.2003.09.003] [PMID]
- Di Nardo F, Mengarelli A, Maranesi E, Burattini L, Fioretti S. Assessment of the ankle muscle co-contraction during normal gait: a surface electromyography study. Journal of Electromyography and Kinesiology. 2015; 25(2):347-54. [DOI:10.1016/j.jelekin.2014.10.016] [PMID]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clinical Neurophysiology. 2006; 117(1):4-15. [DOI:10.1016/j.clinph.2005.08.014] [PMID]
- Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis and Rheumatism. 2007; 57(6):1018-26. [DOI:10.1002/art.22889] [PMID]
- Jafarnezhadgero A, Fatollahi A, Sheykholeslami A, Dionisio VC, Akrami M. Long-term training on sand changes lower limb muscle activities during running in runners with over-pronated feet. Biomedical Engineering Online. 2021; 20(1):118.[DOI:10.1186/s12938-021-00955-8] [PMID]
- Jafarnezhadgero AA, Fatollahi A, Granacher U. Eight weeks of exercising on sand has positive effects on biomechanics of walking and muscle activities in individuals with pronated feet: A randomized double-blinded controlled trial. Sports. 2022; 10(5):70. [DOI:10.3390/sports10050070] [PMID]
- Hwang BH, Kim TH. The effects of sand surface training on changes in the muscle activity of the paretic side lower limb and the improvement of dynamic stability and gait endurance in stroke patients. Journal of Exercise Rehabilitation. 2019; 15(3):439-44. [DOI:10.12965/jer.1938164.082] [PMID]
- Jafarnezhadgero A, Fatollahi A, Amirzadeh N, Siahkouhian M, Granacher U. Ground reaction forces and muscle activity while walking on sand versus stable ground in individuals with pronated feet compared with healthy controls. Plos One. 2019; 14(9):e0223219. [DOI:10.1371/journal.pone.0223219] [PMID]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, et al. European recommendations for surface electromyography. Roessingh Research and Development. 1999; 8(2):13-54. [Link]
- Heiden TL, Lloyd DG, Ackland TR. Knee joint kinematics, kinetics and muscle co-contraction in knee osteoarthritis patient gait. Clinical Biomechanics. 2009; 24(10):833-41. [DOI:10.1016/j.clinbiomech.2009.08.005] [PMID]
- Durai DBJ, Shaju MF. Effect of sand running training on speed among school boys. International Journal of Physical Education, Sports and Health. 2019; 6(3):117-22. [Link]
- Apps C, Sterzing T, O'Brien T, Lake M. Lower limb joint stiffness and muscle co-contraction adaptations to instability footwear during locomotion. Journal of Electromyography and Kinesiology. 2016; 31:55-62. [DOI:10.1016/j.jelekin.2016.09.003] [PMID]
- Jeong JH. Neuromechanical effects of pronated foot on knee joint stability [Msc thesis]. Seoul: Yonsei University; 2011. [Link]
- Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D'Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. The American Journal of Sports Medicine. 1988; 16(2):113-22. [DOI:10.1177/036354658801600205] [PMID]
- Li Y, Ko J, Walker MA, Brown CN, Schmidt JD, Kim SH, Simpson KJ. Does chronic ankle instability influence lower extremity muscle activation of females during landing? Journal of Electromyography and Kinesiology. 2018; 38:81-87. [DOI:10.1016/j.jelekin.2017.11.009] [PMID]
- Fong DT, Hong Y, Li JX. Lower-extremity gait kinematics on slippery surfaces in construction worksites. Medicine and Science in Sports and Exercise. 2005; 37(3):447-54. [DOI:10.1249/01.MSS.0000155390.41572.DE] [PMID]
- Chmielewski TL, Hurd WJ, Rudolph KS, Axe MJ, Snyder-Mackler L. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Physical Therapy. 2005; 85(8):740-9. [DOI:10.1093/ptj/85.8.740] [PMID]
- Ferris DP, Louie M, Farley CT. Running in the real world: Adjusting leg stiffness for different surfaces. Proceedings. Biological Sciences. 1998; 265(1400):989-94. [DOI:10.1098/rspb.1998.0388] [PMID]
- Hodges PW, Tucker K. Moving differently in pain: A new theory to explain the adaptation to pain. Pain. 2011; 152(3 Suppl):S90-8. [DOI:10.1016/j.pain.2010.10.020] [PMID]
- Hodges PW, van den Hoorn W, Wrigley TV, Hinman RS, Bowles KA, Cicuttini F, et al. Increased duration of co-contraction of medial knee muscles is associated with greater progression of knee osteoarthritis. Manual Therapy. 2016; 21:151-8. [DOI:10.1016/j.math.2015.07.004] [PMID]
- Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis and Cartilage. 2004; 12(9):745-51. [DOI:10.1016/j.joca.2004.05.005] [PMID]
- Felson DT, Hannan MT, Naimark A, Berkeley J, Gordon G, Wilson PW, et al. Occupational physical demands, knee bending, and knee osteoarthritis: Results from the Framingham Study. The Journal of Rheumatology. 1991; 18(10):1587-92. [PMID]
- Hubley-Kozey CL, Hill NA, Rutherford DJ, Dunbar MJ, Stanish WD. Co-activation differences in lower limb muscles between asymptomatic controls and those with varying degrees of knee osteoarthritis during walking. Clinical Biomechanics. 2009; 24(5):407-14. [DOI:10.1016/j.clinbiomech.2009.02.005] [PMID]
- Höhne A, Ali S, Stark C, Brüggemann GP. Reduced plantar cutaneous sensation modifies gait dynamics, lower-limb kinematics and muscle activity during walking. European Journal of Applied Physiology. 2012; 112(11):3829-38. [DOI:10.1007/s00421-012-2364-2] [PMID]
- Laibsirinon S. Impact peak, knee muscle cocontraction, and knee angle during early contact of running in children with diplegic cerebral palsy [PhD dissertation]. Philadelphia: Drexel University; 2016. [Link]
Type of Study: Research |
Subject:
Sport injury and corrective exercises
Received: 2023/06/11 | Accepted: 2023/07/18 | Published: 2023/10/14
Received: 2023/06/11 | Accepted: 2023/07/18 | Published: 2023/10/14
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






