Mon, Nov 24, 2025
Volume 8, Issue 2 (Summer 2018)
PTJ 2018, 8(2): 63-76 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahiraei S, Daneshmandi H, Norasteh A A, Sokhangoei Y. The Study of Biomechanical Gait Cycle and Balance Characteristics in Intellectual Disabilities: A Systematic Review. PTJ 2018; 8 (2) :63-76
URL: http://ptj.uswr.ac.ir/article-1-360-en.html
URL: http://ptj.uswr.ac.ir/article-1-360-en.html
1- Department of Sport Injury and Corrective Exercises, School of Physical Education and Sport Sciences, University of Guilan, Rasht, Iran.
2- Department of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
Full-Text [PDF 613 kb]
(2058 Downloads)
| Abstract (HTML) (6514 Views)
Kinematic characteristics
Several studies have investigated the kinematic characteristics of gait in ID patients. These studies were carried out in motion analysis laboratories using motion analysis systems such as optoelectronic system, video recording, and GAITRite system with markers on anatomical landmarks. ID patients with DS demonstrated higher mean pelvic tilt and Range of Motion (ROM) of the pelvis on sagittal and frontal planes during the gait cycle compared to their typically developed peers [16, 17].
In hip joint, higher knee angle in the sagittal plane at the initial contact has been reported. Higher values for minimal hip flexion and maximum hip extension in stance phase and increased hip ROM on the frontal plane have also been reported for ID patients [16, 17, 19, 20]. Another study reported lower ROM values of hip flexion-extension in the sagittal plane. The values of knee angle at the initial contact in the sagittal plane for DS patients were higher compared to their typically developed peers [17, 20]. Studies reporting higher values of minimal knee flexion in the stance phase among DS patients were also available.
They have indicated the lower ROM values of knee flexion-extension and knee maximum flexion in swing phase [16, 17, 19]. For ankle joint kinematics in ID patients, the lower values of ankle angle in the sagittal plane at the initial contact and ROM of ankle dorsi-plantar flexion were also found [16, 17, 19, 20]. In addition, lower values for maximum ankle dorsiflexion at stance and swing phases as well as plantar flexion at the end of the stance phase were found. Kim et al. reported high external rotation in DS patients compared to their healthy peers [12].
In terms of the shoulder joint, Rigoldi et al. observed higher ROM in the frontal plane in a gait cycle in children, adolescents, and adults with DS [19]. Studies on people with DS suggested fast gait with higher cadence, shorter steps, higher double-support phase, and a wider stance in them. Rigoldi et al. observed gait quality and reduced cerebellar vermis volume in people with DS. They also reported an association between the prevalence of gait asymmetry and the reduction of gray matter volume [21].
In patients with PWS, higher values of pelvic tilt, hip flexion angle at the initial contact in the sagittal plane, minimal hip flexion in stance phase, and ROM of hip flexion-extension were documented [15, 16]. In respect of the knee joint of PWS patients, higher values of knee angle at the initial contact in the sagittal plane were reported [16]. In addition, lower values of knee flexion-extension ROM, minimal knee flexion in stance phase, and maximum knee flexion in swing phase were reported [15, 16].
For ankle joint of PWS patients, lower ankle angle at the initial contact in the sagittal plane was reported, as well as lower values for minimum and maximum ankle dorsiflexion in stance phase, and low ROM of ankle dorsi-plantar flexion. However, maximum ankle dorsiflexion at the swing phase was higher [16]. Haynes and Lockhart argued that ID adults had higher values of knee angle at the initial contact with the ground. However, they were not different from their healthy peers in terms of ankle angle at the initial contact [22].
Kinetic characteristics
Some studies were found on the kinetic characteristics of gait in ID patients. These studies only evaluated DS and PWS patients using treadmills or force plates. In comparison with healthy peers, higher first ankle peak force and lower second ankle peak force were reported in ID patients [23, 24].
Reduced ankle power generation and ground reaction force in mediolateral and anterior-posterior planes were observed in these patients, too [12, 17, 19, 20]. In patients with PWS, Vismara et al. reported the low peak of plantar flexion moment and the low peak of ankle generated power [15]. Additionally, the peak of ankle power at the end of the stance phase was higher in these patients [16].
Balance characteristics of ID patients
Studies indicate that at steady state, the sway amplitude varies in subjects with ID, compared to the controls. People with ID also show a lateral oscillatory pattern, where increased sway in the anterior part of the frontal plane is higher than that in the sagittal plane. No significant correlation has been observed between sagittal/lateral sways and Intelligence Quotient (IQ) in ID subjects.
In a group of people with DS, sway amplitudes were not consistently greater than that of the control group, but they showed high rates of sway. Carmeli et al. indicated that the oscillation amplitude between eyes-closed and eyes-open resting conditions is higher in normal people compared to ID patients [31]. Most studies have demonstrated that individuals with ID have lower balance capacity and reduced ability to respond to external balance perturbations. According to Lahtinen et al. adolescents and adults with ID have impaired static balance, measured by the Stork balance test.
Carvalho and Almeida measured the longest time on a seesaw in persons with DS who were 21 years younger than the control counterparts (i.e. 28 VS. 49 years old). They found no difference between the groups [33]. Similarly, people with ID [34] and DS [6] showed decreased performance in the single leg stance test. Furthermore, individuals with ID [10] or DS [13] scored better in the Berg balance scale compared to their non-symptomatic peers. Posturography measures have suggested that patients with ID or DS have high sway amplitude and variability in comparison with controls. In addition, their balance strategy is based on shifting the body weight mediolaterally (Table 2).
Comparing individuals with ID, DS, and healthy peers revealed that ID patients have significantly more body sway than the DS and healthy individuals. Also, those with DS had significant differences with their healthy peers. Moreover, a significant correlation between the increased sway and the severity of disease was reported. However, a recent study argues that the severity of this disease has a negative effect on static and dynamic balance in children with ID. Thus, this characteristic remains controversial. Rigoldi et al. examined balance ability using posturography in three different age groups with and without DS, and found that they use different strategies, based on their age [19].
Children (aged 9-11 years) with DS had increased ROM of the Center of Pressure (COP) on the anteroposterior and mediolateral planes and increased trajectory length of the COP. Adolescents (aged 12-19 years) with DS had increased frequency in the anteroposterior and mediolateral deviation of the COP and increased ROM of the COP on the anteroposterior plane. Adults (aged 22-46 years) with DS showed high ROM of the COP on the anteroposterior and mediolateral planes and high frequency in the deviation of the COP on the anteroposterior axis [33].
There is no consensus among researchers regarding the importance of visual cues on balance, according to the degree of deterioration in eyes-closed balance. Many scholars have argued that people with ID and DS have higher body sway in eyes-closed condition, compared to the control groups. However, the findings of Dellavia et al. and Gomes and Barela [35, 36] are inconsistent with these results.
4. Discussion
Balance and gait characteristics of people with ID were reviewed in this paper. The results suggest that gait abnormalities and balance disorders in the ID population with and without genetic syndrome are evident. In comparison with healthy counterparts, ID people show more instability in balance and gait. In addition, they show an increased level of antagonistic co-contraction due to muscle stiffness during standing and walking. Walking in ID people is slower and asymmetrical with wider and shorter strides. The literature review reveals that most studies have explored spatiotemporal gait parameters, and less attention have been paid to kinematic characteristics. Only a few studies were found on the kinetic gait characteristics of ID patients which were conducted on DS and PWS groups.
Full-Text: (3056 Views)
1. Introduction
According to the American Association on Intellectual and Developmental Disabilities, Intellectual Disability (ID) refers to conditions where intellectual functioning is clearly less than the average level. ID also refers to concurrent limitations in adaptive behavior during the growth period before the age of 18. Its average worldwide prevalence range has been reported to be 1 to 3% [1]. Meanwhile, mild, moderate, severe, and profound IDs are observed in 85%, 10%, 4%, and 2% of the total population, respectively [2].
ID is associated with certain physio-anatomical features, distinguishing its sufferers from the normal population. For example, the ID patients’ brain is lighter and smaller, they have decreased neuronal density, and owning to reduced neurotransmitters, they have synaptic dysfunction. Myelination abnormalities, reduced muscle tension, ligamentous laxity, vision and hearing impairments, inner ear neurosensory disorders, cardiovascular diseases, respiratory problems, etc., as well as appearance-related features, are other characteristics of ID [3-5].
Independent and secure participation in society and daily life activities is very important. Pathoneurological causes of motor impairment in patients with ID are unrecognized. However, cerebellar dysfunction, delayed myelination, vestibular disorders, proprioception, decreased muscle strength and tension, ligamentous laxity and visual impairments have been reported as the causes of this disorder [6]. Several mechanisms may cause balance and gait problems in ID patients. First, the incomplete development of mind which affects motor-cognitive function [7].
The second mechanism is premature aging. Balance and gait deterioration in these patients is related to aging because of decreased muscle strength and sensory function (vision, proprioception & vestibular system). Age-related problems in ID patients are largely similar to the normal people. However, they occur more frequently and at younger ages in ID patients [8]. Additionally, given that life expectancy has increased, the number of older people with ID, as well as those with balance and gait problems is rapidly growing [9]. The third mechanism is lifestyle. ID patients are generally inactive [10]. As a result, their physical capacities such as endurance, balance, and strength are less than their healthy counterparts.
Walking has been recognized as a cognitive function. Gait and balance refer to the integration of attention, planning, memory, and other motor, perceptual, and cognitive processes [11]. Gait and balance require efficiency, endurance, and spatiotemporal accuracy, which depending on gross motor skills, are acquired during the early stages of development. Any disruption in the development of these motor skills reduces individual autonomy and calls for constant support in daily activities [11].
Relative limitation in the joints, as well as weakness in the lower limb muscles, can disrupt such development process. Functional limitations of ID patients may be low. Thus, a correct walking pattern may be observed along with an impaired sensorimotor integration. This can cause reduced gait speed, larger cadence, a shorter stride and step length, larger step width, greater double leg support time, reduced lower limb joints mobility, biomechanical changes and adopting the strategy of “caution in taking steps”, because of the fear of falling [12]. Considering motor impairments in these patients and the available reports on their gait and balance problems, this research aims to explore the biomechanical gait cycle and balance characteristics of ID patients.
2. Materials and Methods
This was a systematic review of studies published online from 1992 to 2017. The keywords for the literature search used individually or together were “gait”, “balance”, and “intellectual disability”. For detailed search, the following keywords were also used: “spatiotemporal gait parameters”, “kinetics and kinematics of gait” ,“gait speed”, “cadence”, “stride length”, “step width”, “static balance”, “dynamic balance”, “postural control and postural stability”.
The online international databases for literature search were PubMed, EMBASE, Cochrane, TRIP, PEDro, MedLib, CINAHL, ProQuest, and Google Scholar. Additionally, the Iranian online databases included SID, Magiran, Irandoc, and IranMedex were searched too. At the initial screening stage, only the title and abstract of the candidate studies were considered for deciding on whether or not be included in the study in our review.
Inclusion criteria were studies on gait and balance of ID patients conducted during 1992-2017, published in English or Persian scientific research journals with full text available. Moreover, the exclusion criteria consisted of the lack of access to full texts, studying gait and balance of disabilities other than ID, and abstracts presented at conferences. A data extraction form was designed based on the research purpose. The selected studies were 56, of which 20 were removed due to not meeting the inclusion criteria. Finally, 36 papers were reviewed. Of them, 16 were related to biomechanical gait cycle characteristics, and 20 were about the balance characteristics of ID patients. Figure 1 illustrates the process of selecting candidate studies for review.
3. Results
Gait cycle characteristics of ID patients
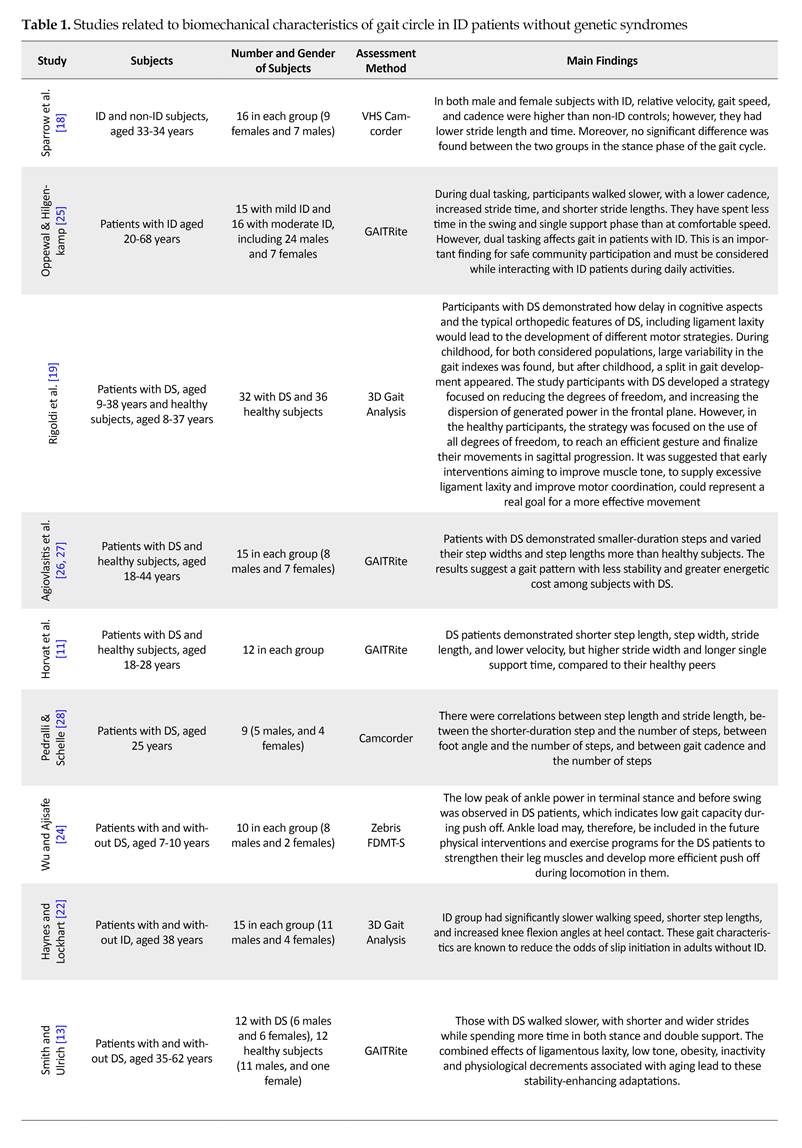
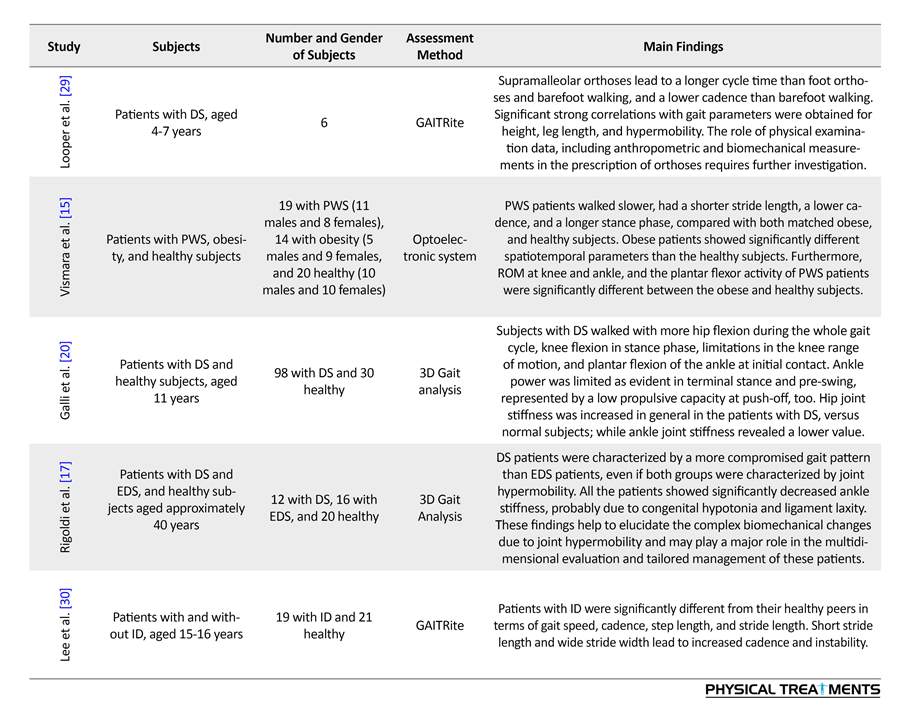
Table 1 presents the studies examining the biomechanical characteristics of the gait circle in ID patients during 1992-2017.
Spatiotemporal parameters
Most studies have examined spatiotemporal gait parameters of ID patients, including stride length, stride and step width, step length, velocity, cadence, Single Support Time (SST), Double Support Time (DST), and the base of support. In most studies, GAITRite system was used for spatiotemporal gait analysis, and treadmill to assess the walking speed. We applied a three-dimensional optoelectronic motion analysis system to measure spatiotemporal parameters.
The studies indicate that ID patients have a lower swing, step and stride length, speed, and cadence, compared to their normal peers. In contrast, Horvat et al. reported higher step and stride length in them [11]. Additionally, Smith et al. suggested a higher cadence in ID patients [13]. Most studies have reported higher step and stride width, the base of support, stance time percentage, stance time, DST percentage, and DST in ID patients. However, Gretz et al. [14] reported shorter step time and Horvat et al. [11] documented their lower scores in step width and DST.
Regarding other genetic syndromes, there were studies on patients with Prader-Willi Syndrome (PWS), reporting their higher scores in velocity and DST, and their lower scores in cadence, stride length and stance time as a percentage of gait cycle [15, 16]. In one study, patients with Ehlers-Danlos syndrome were examined who had a longer stride, higher cadence, and shorter DST as a percentage of gait cycle [17].
For the ID people without syndromes, low gait speeds and step lengths have been reported in comparison with healthy counterparts. However, Sparrow et al. found higher gait speed and cadence, and shorter step and stride length in both male and female patients [18]. In general, it seems that ID patients walk faster and take longer strides in a shorter time. In addition, they have a lower base of support and shorter DST in a gait cycle, in comparison with ID patients with Down Syndrome (DS), indicating their better walking pattern.
According to the American Association on Intellectual and Developmental Disabilities, Intellectual Disability (ID) refers to conditions where intellectual functioning is clearly less than the average level. ID also refers to concurrent limitations in adaptive behavior during the growth period before the age of 18. Its average worldwide prevalence range has been reported to be 1 to 3% [1]. Meanwhile, mild, moderate, severe, and profound IDs are observed in 85%, 10%, 4%, and 2% of the total population, respectively [2].
ID is associated with certain physio-anatomical features, distinguishing its sufferers from the normal population. For example, the ID patients’ brain is lighter and smaller, they have decreased neuronal density, and owning to reduced neurotransmitters, they have synaptic dysfunction. Myelination abnormalities, reduced muscle tension, ligamentous laxity, vision and hearing impairments, inner ear neurosensory disorders, cardiovascular diseases, respiratory problems, etc., as well as appearance-related features, are other characteristics of ID [3-5].
Independent and secure participation in society and daily life activities is very important. Pathoneurological causes of motor impairment in patients with ID are unrecognized. However, cerebellar dysfunction, delayed myelination, vestibular disorders, proprioception, decreased muscle strength and tension, ligamentous laxity and visual impairments have been reported as the causes of this disorder [6]. Several mechanisms may cause balance and gait problems in ID patients. First, the incomplete development of mind which affects motor-cognitive function [7].
The second mechanism is premature aging. Balance and gait deterioration in these patients is related to aging because of decreased muscle strength and sensory function (vision, proprioception & vestibular system). Age-related problems in ID patients are largely similar to the normal people. However, they occur more frequently and at younger ages in ID patients [8]. Additionally, given that life expectancy has increased, the number of older people with ID, as well as those with balance and gait problems is rapidly growing [9]. The third mechanism is lifestyle. ID patients are generally inactive [10]. As a result, their physical capacities such as endurance, balance, and strength are less than their healthy counterparts.
Walking has been recognized as a cognitive function. Gait and balance refer to the integration of attention, planning, memory, and other motor, perceptual, and cognitive processes [11]. Gait and balance require efficiency, endurance, and spatiotemporal accuracy, which depending on gross motor skills, are acquired during the early stages of development. Any disruption in the development of these motor skills reduces individual autonomy and calls for constant support in daily activities [11].
Relative limitation in the joints, as well as weakness in the lower limb muscles, can disrupt such development process. Functional limitations of ID patients may be low. Thus, a correct walking pattern may be observed along with an impaired sensorimotor integration. This can cause reduced gait speed, larger cadence, a shorter stride and step length, larger step width, greater double leg support time, reduced lower limb joints mobility, biomechanical changes and adopting the strategy of “caution in taking steps”, because of the fear of falling [12]. Considering motor impairments in these patients and the available reports on their gait and balance problems, this research aims to explore the biomechanical gait cycle and balance characteristics of ID patients.
2. Materials and Methods
This was a systematic review of studies published online from 1992 to 2017. The keywords for the literature search used individually or together were “gait”, “balance”, and “intellectual disability”. For detailed search, the following keywords were also used: “spatiotemporal gait parameters”, “kinetics and kinematics of gait” ,“gait speed”, “cadence”, “stride length”, “step width”, “static balance”, “dynamic balance”, “postural control and postural stability”.
The online international databases for literature search were PubMed, EMBASE, Cochrane, TRIP, PEDro, MedLib, CINAHL, ProQuest, and Google Scholar. Additionally, the Iranian online databases included SID, Magiran, Irandoc, and IranMedex were searched too. At the initial screening stage, only the title and abstract of the candidate studies were considered for deciding on whether or not be included in the study in our review.
Inclusion criteria were studies on gait and balance of ID patients conducted during 1992-2017, published in English or Persian scientific research journals with full text available. Moreover, the exclusion criteria consisted of the lack of access to full texts, studying gait and balance of disabilities other than ID, and abstracts presented at conferences. A data extraction form was designed based on the research purpose. The selected studies were 56, of which 20 were removed due to not meeting the inclusion criteria. Finally, 36 papers were reviewed. Of them, 16 were related to biomechanical gait cycle characteristics, and 20 were about the balance characteristics of ID patients. Figure 1 illustrates the process of selecting candidate studies for review.
3. Results
Gait cycle characteristics of ID patients
Table 1 presents the studies examining the biomechanical characteristics of the gait circle in ID patients during 1992-2017.
Spatiotemporal parameters
Most studies have examined spatiotemporal gait parameters of ID patients, including stride length, stride and step width, step length, velocity, cadence, Single Support Time (SST), Double Support Time (DST), and the base of support. In most studies, GAITRite system was used for spatiotemporal gait analysis, and treadmill to assess the walking speed. We applied a three-dimensional optoelectronic motion analysis system to measure spatiotemporal parameters.
The studies indicate that ID patients have a lower swing, step and stride length, speed, and cadence, compared to their normal peers. In contrast, Horvat et al. reported higher step and stride length in them [11]. Additionally, Smith et al. suggested a higher cadence in ID patients [13]. Most studies have reported higher step and stride width, the base of support, stance time percentage, stance time, DST percentage, and DST in ID patients. However, Gretz et al. [14] reported shorter step time and Horvat et al. [11] documented their lower scores in step width and DST.
Regarding other genetic syndromes, there were studies on patients with Prader-Willi Syndrome (PWS), reporting their higher scores in velocity and DST, and their lower scores in cadence, stride length and stance time as a percentage of gait cycle [15, 16]. In one study, patients with Ehlers-Danlos syndrome were examined who had a longer stride, higher cadence, and shorter DST as a percentage of gait cycle [17].
For the ID people without syndromes, low gait speeds and step lengths have been reported in comparison with healthy counterparts. However, Sparrow et al. found higher gait speed and cadence, and shorter step and stride length in both male and female patients [18]. In general, it seems that ID patients walk faster and take longer strides in a shorter time. In addition, they have a lower base of support and shorter DST in a gait cycle, in comparison with ID patients with Down Syndrome (DS), indicating their better walking pattern.
Kinematic characteristics
Several studies have investigated the kinematic characteristics of gait in ID patients. These studies were carried out in motion analysis laboratories using motion analysis systems such as optoelectronic system, video recording, and GAITRite system with markers on anatomical landmarks. ID patients with DS demonstrated higher mean pelvic tilt and Range of Motion (ROM) of the pelvis on sagittal and frontal planes during the gait cycle compared to their typically developed peers [16, 17].
In hip joint, higher knee angle in the sagittal plane at the initial contact has been reported. Higher values for minimal hip flexion and maximum hip extension in stance phase and increased hip ROM on the frontal plane have also been reported for ID patients [16, 17, 19, 20]. Another study reported lower ROM values of hip flexion-extension in the sagittal plane. The values of knee angle at the initial contact in the sagittal plane for DS patients were higher compared to their typically developed peers [17, 20]. Studies reporting higher values of minimal knee flexion in the stance phase among DS patients were also available.
They have indicated the lower ROM values of knee flexion-extension and knee maximum flexion in swing phase [16, 17, 19]. For ankle joint kinematics in ID patients, the lower values of ankle angle in the sagittal plane at the initial contact and ROM of ankle dorsi-plantar flexion were also found [16, 17, 19, 20]. In addition, lower values for maximum ankle dorsiflexion at stance and swing phases as well as plantar flexion at the end of the stance phase were found. Kim et al. reported high external rotation in DS patients compared to their healthy peers [12].
In terms of the shoulder joint, Rigoldi et al. observed higher ROM in the frontal plane in a gait cycle in children, adolescents, and adults with DS [19]. Studies on people with DS suggested fast gait with higher cadence, shorter steps, higher double-support phase, and a wider stance in them. Rigoldi et al. observed gait quality and reduced cerebellar vermis volume in people with DS. They also reported an association between the prevalence of gait asymmetry and the reduction of gray matter volume [21].
In patients with PWS, higher values of pelvic tilt, hip flexion angle at the initial contact in the sagittal plane, minimal hip flexion in stance phase, and ROM of hip flexion-extension were documented [15, 16]. In respect of the knee joint of PWS patients, higher values of knee angle at the initial contact in the sagittal plane were reported [16]. In addition, lower values of knee flexion-extension ROM, minimal knee flexion in stance phase, and maximum knee flexion in swing phase were reported [15, 16].
For ankle joint of PWS patients, lower ankle angle at the initial contact in the sagittal plane was reported, as well as lower values for minimum and maximum ankle dorsiflexion in stance phase, and low ROM of ankle dorsi-plantar flexion. However, maximum ankle dorsiflexion at the swing phase was higher [16]. Haynes and Lockhart argued that ID adults had higher values of knee angle at the initial contact with the ground. However, they were not different from their healthy peers in terms of ankle angle at the initial contact [22].
Kinetic characteristics
Some studies were found on the kinetic characteristics of gait in ID patients. These studies only evaluated DS and PWS patients using treadmills or force plates. In comparison with healthy peers, higher first ankle peak force and lower second ankle peak force were reported in ID patients [23, 24].
Reduced ankle power generation and ground reaction force in mediolateral and anterior-posterior planes were observed in these patients, too [12, 17, 19, 20]. In patients with PWS, Vismara et al. reported the low peak of plantar flexion moment and the low peak of ankle generated power [15]. Additionally, the peak of ankle power at the end of the stance phase was higher in these patients [16].
Balance characteristics of ID patients
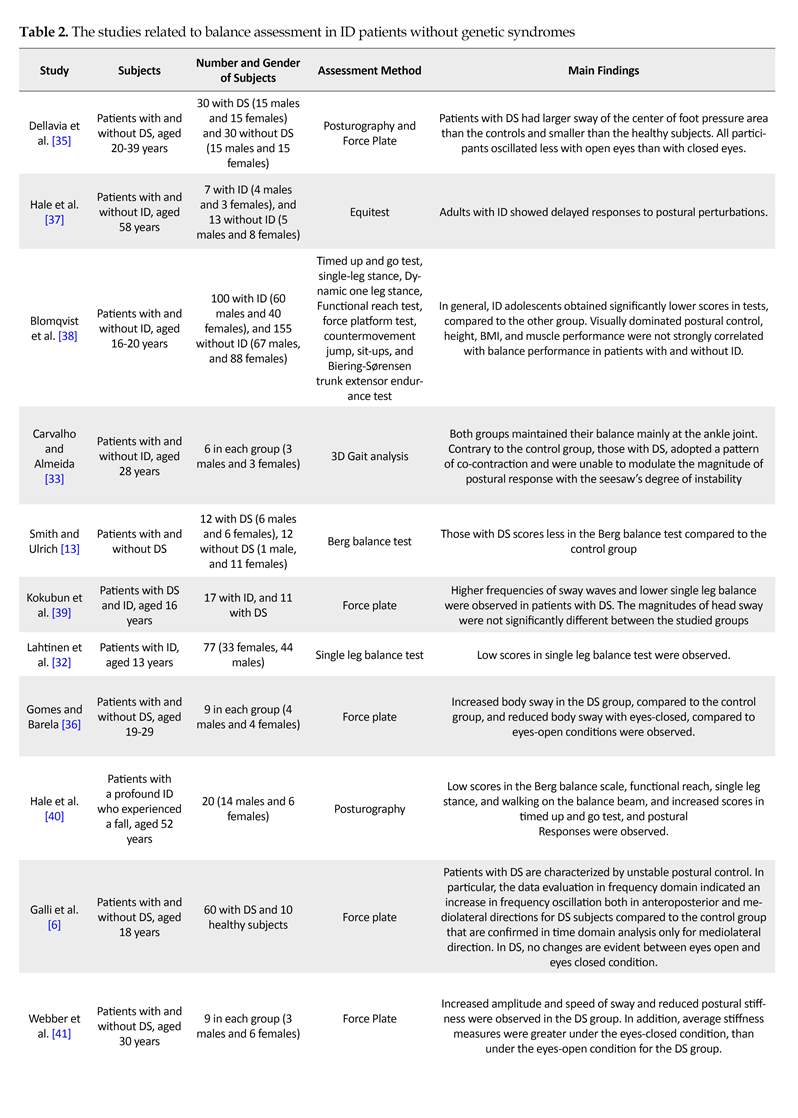
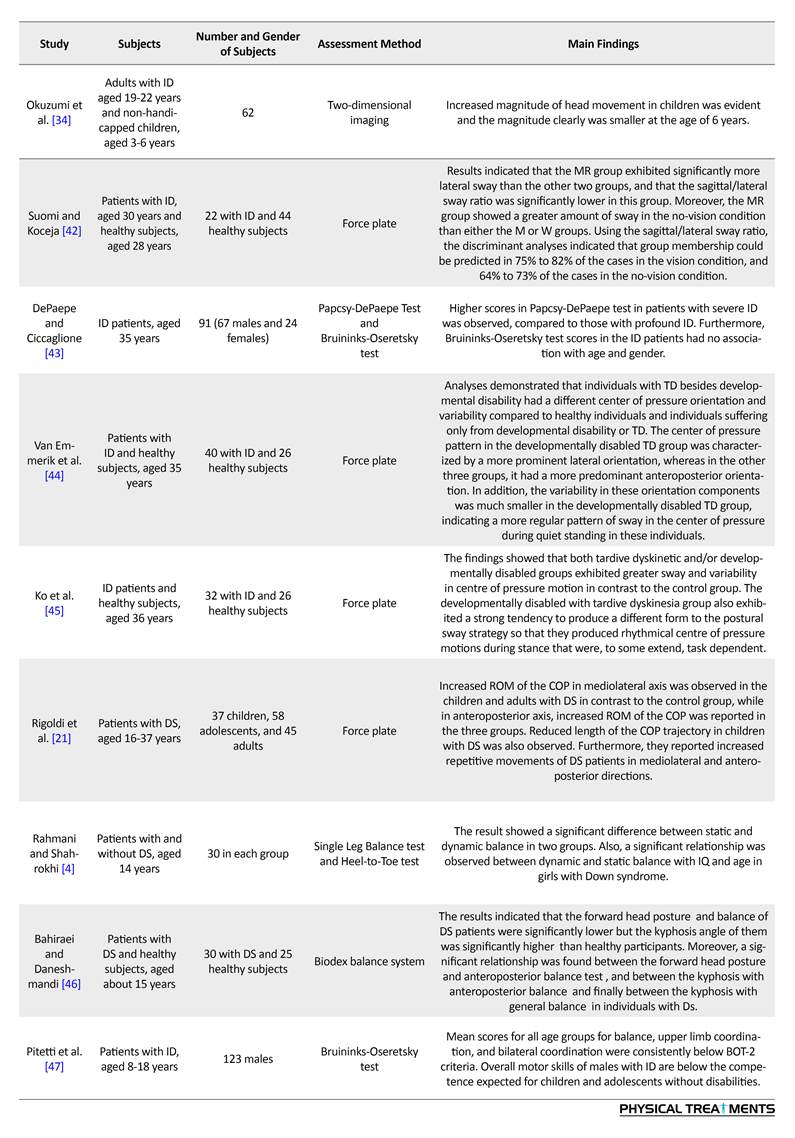
Studies indicate that at steady state, the sway amplitude varies in subjects with ID, compared to the controls. People with ID also show a lateral oscillatory pattern, where increased sway in the anterior part of the frontal plane is higher than that in the sagittal plane. No significant correlation has been observed between sagittal/lateral sways and Intelligence Quotient (IQ) in ID subjects.
In a group of people with DS, sway amplitudes were not consistently greater than that of the control group, but they showed high rates of sway. Carmeli et al. indicated that the oscillation amplitude between eyes-closed and eyes-open resting conditions is higher in normal people compared to ID patients [31]. Most studies have demonstrated that individuals with ID have lower balance capacity and reduced ability to respond to external balance perturbations. According to Lahtinen et al. adolescents and adults with ID have impaired static balance, measured by the Stork balance test.
Carvalho and Almeida measured the longest time on a seesaw in persons with DS who were 21 years younger than the control counterparts (i.e. 28 VS. 49 years old). They found no difference between the groups [33]. Similarly, people with ID [34] and DS [6] showed decreased performance in the single leg stance test. Furthermore, individuals with ID [10] or DS [13] scored better in the Berg balance scale compared to their non-symptomatic peers. Posturography measures have suggested that patients with ID or DS have high sway amplitude and variability in comparison with controls. In addition, their balance strategy is based on shifting the body weight mediolaterally (Table 2).
Comparing individuals with ID, DS, and healthy peers revealed that ID patients have significantly more body sway than the DS and healthy individuals. Also, those with DS had significant differences with their healthy peers. Moreover, a significant correlation between the increased sway and the severity of disease was reported. However, a recent study argues that the severity of this disease has a negative effect on static and dynamic balance in children with ID. Thus, this characteristic remains controversial. Rigoldi et al. examined balance ability using posturography in three different age groups with and without DS, and found that they use different strategies, based on their age [19].
Children (aged 9-11 years) with DS had increased ROM of the Center of Pressure (COP) on the anteroposterior and mediolateral planes and increased trajectory length of the COP. Adolescents (aged 12-19 years) with DS had increased frequency in the anteroposterior and mediolateral deviation of the COP and increased ROM of the COP on the anteroposterior plane. Adults (aged 22-46 years) with DS showed high ROM of the COP on the anteroposterior and mediolateral planes and high frequency in the deviation of the COP on the anteroposterior axis [33].
There is no consensus among researchers regarding the importance of visual cues on balance, according to the degree of deterioration in eyes-closed balance. Many scholars have argued that people with ID and DS have higher body sway in eyes-closed condition, compared to the control groups. However, the findings of Dellavia et al. and Gomes and Barela [35, 36] are inconsistent with these results.
4. Discussion
Balance and gait characteristics of people with ID were reviewed in this paper. The results suggest that gait abnormalities and balance disorders in the ID population with and without genetic syndrome are evident. In comparison with healthy counterparts, ID people show more instability in balance and gait. In addition, they show an increased level of antagonistic co-contraction due to muscle stiffness during standing and walking. Walking in ID people is slower and asymmetrical with wider and shorter strides. The literature review reveals that most studies have explored spatiotemporal gait parameters, and less attention have been paid to kinematic characteristics. Only a few studies were found on the kinetic gait characteristics of ID patients which were conducted on DS and PWS groups.
Children and adults with DS demonstrate the general characteristics of walking with reduced stability, reflected by the displacement of the Center of Mass (COM). Specifically, reduced stability shown in adults with DS is documented by the increased variability in the base of stance width and step length by the increased mediolateral variability of the COM. It is also demonstrated by the higher mediolateral ROM of the COM, especially at higher gait speeds. Similar findings have been reported in studies on children with ID during the gait cycle [4].
The similarity of measures for assessing gait and balance characteristics in ID patients (such as posturography and gait analysis), allows the comparison of normal children. Thus, they can be used as a scale for examining growth retardation. Besides age, such similarities appear to be dependent on the IQ and frequency of motor skills experiences of these individuals [25].
ID groups are widely heterogeneous; therefore, there are various explanations for gait abnormalities which include their physio-cognitive specifications. Physical features, especially in ID people with genetic syndromes, have received more attention among researchers. For example, in those with DS, hypermobility has been reported to have a significant biomechanical effect on the gait pattern of these individuals due to ligamentous laxity and hypotonia which may explain the abnormalities of gait in DS people [11, 17, 20].
In people with PWS, slower walking, shorter stride length, lower cadence, and longer stance phase have been observed. In addition, mediolateral variability of the COM may be because of severe obesity, low muscle strength, hypotonia and small feet [15]. All of these characteristics lead to a cautious walking pattern for maintaining stability during a gait cycle. However, gait abnormalities in individuals with ID, regardless of their syndrome and specific physical characteristics, cannot clearly explain all the disorders associated with these people. Therefore, the relationship between gait and the cognitive level is important. Such a relationship has been investigated in older adults with normal cognition and cognitive deficits. It was found that specific executive cognitive deficits would account for slower usual gait in aging [48].
Targeted cognitive interventions can improve gait performance [49]. For specific cognitive domains, it has been shown that the processing speed has been linked to temporal gait variables. This is while walking performance has been associated with spatial gait variables. Holtzer et al. reported that executive attention, memory, and verbal IQ are related to velocity. Gait abnormalities such as high gait variability and low velocity are related to cognitive decline, mild cognitive impairment and Alzheimer disease in the elderly [50].
One study evaluated the relationship between cognitive components with gait and balance in individuals with ID by performing dual tasking and walking over obstacles. Their obtained results indicated that, in the framework of walking and balance, it also has an additional cognitive challenge [51]. Under such conditions, it was revealed that participants increase their gait by stability adaptations, such as reducing velocity and increasing stride length and width. This cognitive component has received less attention in investigating the gait abnormalities in ID individuals.
In terms of falling associated with gait and balance, it can be argued that in addition to gait disturbance, it causes high energy costs [52]. It seems that mechanisms related to gait patterns such as hip joint extension, stride width, and cadence are related to energy cost in the elderly and gait variables [53]. In people with ID, gait variability leads to high oxygen uptake, increased heart rate, and a non-economic gait. Walking under perturbations and over obstacles adds additional stress to various cognitive abilities.
This forces the brain to perform another action before walking. Thus, changes in gait parameters (e.g. reducing velocity) can be a strategy before other actions. In the elderly, mild cognitive impairments and Alzheimer disease or dementia may worsen walking. Therefore, some disorders are correlated with certain cognitive domains such as dementia, cognitive impairment, and gait abnormalities which often coexist [50].
In ID people, age-related balance impairment is affected by the growth of motor reflex pathways, which regulate muscle tone and control posture. Studies reported that children with mild ID had lower scores compared to healthy children when performing single leg static balance test. Another factor that can affect balance is the increased variability in upper motor neurons. More specifically, increased variability in movement patterns can reflect higher neural responses in the postural control system to produce larger and faster corrections, especially during instability.
This adaptive process has also been observed in those with ID which can be due to either increased body sway velocity during posture or variations in muscle activation. According to the second hypothesis, a study indicates that ID had a significant effect on muscle activation patterns during the sit-to-stand task. In terms of dynamic balance, backward and forward movements on the base of support during standing on both feet, studies argue that individuals with ID have delayed reactive responses [37, 41]. This delay in reflex or premotor response may have implications in fall prevention. The delayed response can be related to lower physical activity associated with the inability to skillfully respond to balance perturbations.
This can induce anxiety and increase demands on cognitive attention. Increased co-contraction observed in people with ID under different circumstances causing stability and improving safety is another related mechanism. Aging in ID people in relation to their gait or balance characteristics is important. This is because in the elderly, aging is associated with impaired gait and balance [54]. Walking speed is an elderly person’s health index. Walking and balance quality is strongly associated with falling in the adult population [55]. The prevalence of fall injuries in the mentally retarded population is 10.4%. [56].
The balance and gait characteristics of people with ID are different compared to their healthy peers. These characteristics initiate at adolescence and are correlated with aging, which can cause serious problems. Despite the importance of falling in adults with ID, the association and mechanisms of these problems with falling are still unclear. It is important to highlight the role of training interventions in improving and reducing their problems.
Ethical Considerations
Compliance with ethical guidelines
This paper was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1397.021).
Funding
This paper was extracted from a PhD. thesis of Saeid Bahiraei, Department of Sport Injury and Corrective Exercises, School of Physical Education and Sport Sciences, University of Guilan.
Authors contributions
Conceptualization: All authors; Methodology: Saeid Bahiraei and Hassan Daneshmandi; Investigation: All authors; Writing and original draft: Saeid Bahiraei; Writing, reviewing and editing: Hassan Daneshmandi and Ali Asghar Norasteh; Funding acquisition: All authors; Resources: All authors; and Supervision: Hassan Daneshmandi.
Conflict of interest
The authors declare no conflict of interest.
References
The similarity of measures for assessing gait and balance characteristics in ID patients (such as posturography and gait analysis), allows the comparison of normal children. Thus, they can be used as a scale for examining growth retardation. Besides age, such similarities appear to be dependent on the IQ and frequency of motor skills experiences of these individuals [25].
ID groups are widely heterogeneous; therefore, there are various explanations for gait abnormalities which include their physio-cognitive specifications. Physical features, especially in ID people with genetic syndromes, have received more attention among researchers. For example, in those with DS, hypermobility has been reported to have a significant biomechanical effect on the gait pattern of these individuals due to ligamentous laxity and hypotonia which may explain the abnormalities of gait in DS people [11, 17, 20].
In people with PWS, slower walking, shorter stride length, lower cadence, and longer stance phase have been observed. In addition, mediolateral variability of the COM may be because of severe obesity, low muscle strength, hypotonia and small feet [15]. All of these characteristics lead to a cautious walking pattern for maintaining stability during a gait cycle. However, gait abnormalities in individuals with ID, regardless of their syndrome and specific physical characteristics, cannot clearly explain all the disorders associated with these people. Therefore, the relationship between gait and the cognitive level is important. Such a relationship has been investigated in older adults with normal cognition and cognitive deficits. It was found that specific executive cognitive deficits would account for slower usual gait in aging [48].
Targeted cognitive interventions can improve gait performance [49]. For specific cognitive domains, it has been shown that the processing speed has been linked to temporal gait variables. This is while walking performance has been associated with spatial gait variables. Holtzer et al. reported that executive attention, memory, and verbal IQ are related to velocity. Gait abnormalities such as high gait variability and low velocity are related to cognitive decline, mild cognitive impairment and Alzheimer disease in the elderly [50].
One study evaluated the relationship between cognitive components with gait and balance in individuals with ID by performing dual tasking and walking over obstacles. Their obtained results indicated that, in the framework of walking and balance, it also has an additional cognitive challenge [51]. Under such conditions, it was revealed that participants increase their gait by stability adaptations, such as reducing velocity and increasing stride length and width. This cognitive component has received less attention in investigating the gait abnormalities in ID individuals.
In terms of falling associated with gait and balance, it can be argued that in addition to gait disturbance, it causes high energy costs [52]. It seems that mechanisms related to gait patterns such as hip joint extension, stride width, and cadence are related to energy cost in the elderly and gait variables [53]. In people with ID, gait variability leads to high oxygen uptake, increased heart rate, and a non-economic gait. Walking under perturbations and over obstacles adds additional stress to various cognitive abilities.
This forces the brain to perform another action before walking. Thus, changes in gait parameters (e.g. reducing velocity) can be a strategy before other actions. In the elderly, mild cognitive impairments and Alzheimer disease or dementia may worsen walking. Therefore, some disorders are correlated with certain cognitive domains such as dementia, cognitive impairment, and gait abnormalities which often coexist [50].
In ID people, age-related balance impairment is affected by the growth of motor reflex pathways, which regulate muscle tone and control posture. Studies reported that children with mild ID had lower scores compared to healthy children when performing single leg static balance test. Another factor that can affect balance is the increased variability in upper motor neurons. More specifically, increased variability in movement patterns can reflect higher neural responses in the postural control system to produce larger and faster corrections, especially during instability.
This adaptive process has also been observed in those with ID which can be due to either increased body sway velocity during posture or variations in muscle activation. According to the second hypothesis, a study indicates that ID had a significant effect on muscle activation patterns during the sit-to-stand task. In terms of dynamic balance, backward and forward movements on the base of support during standing on both feet, studies argue that individuals with ID have delayed reactive responses [37, 41]. This delay in reflex or premotor response may have implications in fall prevention. The delayed response can be related to lower physical activity associated with the inability to skillfully respond to balance perturbations.
This can induce anxiety and increase demands on cognitive attention. Increased co-contraction observed in people with ID under different circumstances causing stability and improving safety is another related mechanism. Aging in ID people in relation to their gait or balance characteristics is important. This is because in the elderly, aging is associated with impaired gait and balance [54]. Walking speed is an elderly person’s health index. Walking and balance quality is strongly associated with falling in the adult population [55]. The prevalence of fall injuries in the mentally retarded population is 10.4%. [56].
The balance and gait characteristics of people with ID are different compared to their healthy peers. These characteristics initiate at adolescence and are correlated with aging, which can cause serious problems. Despite the importance of falling in adults with ID, the association and mechanisms of these problems with falling are still unclear. It is important to highlight the role of training interventions in improving and reducing their problems.
Ethical Considerations
Compliance with ethical guidelines
This paper was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1397.021).
Funding
This paper was extracted from a PhD. thesis of Saeid Bahiraei, Department of Sport Injury and Corrective Exercises, School of Physical Education and Sport Sciences, University of Guilan.
Authors contributions
Conceptualization: All authors; Methodology: Saeid Bahiraei and Hassan Daneshmandi; Investigation: All authors; Writing and original draft: Saeid Bahiraei; Writing, reviewing and editing: Hassan Daneshmandi and Ali Asghar Norasteh; Funding acquisition: All authors; Resources: All authors; and Supervision: Hassan Daneshmandi.
Conflict of interest
The authors declare no conflict of interest.
References
- Harris JC. Intellectual disability: Understanding its development, causes, classification, evaluation, and treatment. Oxford: Oxford University Press; 2006.
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: A meta-analysis of population-based studies. Research in Developmental Disabilities. 2011; 32(2):419-36. [DOI:10.1016/j.ridd.2010.12.018] [PMID]
- Wu J, Ulrich DA, Looper J, Tiernan CW, Angulo Barroso RM. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with Down syndrome after different treadmill interventions. Experimental Brain Research. 2008; 186(2):261-72. [DOI:10.1007/s00221-007-1230-7] [PMID]
- Rahmani P, Shahrokhi H. The study of static and dynamic balance in mentally retarded female students with and without Down Syndrome (DS). Journal of Sports Medicine. 2012; 2(2):97-113.
- Cabeza Ruiz R, García Massó X, Centeno Prada R, Beas Jiménez J, Colado J, González LM. Time and frequency analysis of the static balance in young adults with Down syndrome. Gait & Posture. 2011; 33(1):23-8. [DOI:10.1016/j.gaitpost.2010.09.014] [PMID]
- Galli M, Rigoldi C, Mainardi L, Tenore N, Onorati P, Albertini G. Postural control in patients with Down syndrome. Disability and Rehabilitation. 2008; 30(17):1274-8. [DOI:10.1080/09638280701610353] [PMID]
- World Health Organization. nternational Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2007.
- Evenhuis HM. Want ik wil nog lang leven: Moderne gezondheidszorg voor mensen met verstandelijke beperkingen: Zoetermeer: Raad voor de Volksgezondheid en Zorg; 2002.
- Janicki M, Dalton AJ, Michael Henderson C, Davidson PW. Mortality and morbidity among older adults with intellectual disability: Health services considerations. Disability and Rehabilitation. 1999; 21(5-6):284-94. [DOI:10.1080/096382899297710] [PMID]
- Hall JM, Thomas MJ. Promoting physical activity and exercise in older adults with developmental disabilities. Topics in Geriatric Rehabilitation. 2008; 24(1):64-73. [DOI:10.1097/01.TGR.0000311407.09178.55]
- Horvat M, Croce R, Zagrodnik J, Brooks B, Carter K. Spatial and temporal variability of movement parameters in individuals with down syndrome. Perceptual and Motor Skills. 2012; 114(3):774-82. [DOI:10.2466/25.15.26.PMS.114.3.774-782] [PMID]
- Kim BS, Bang D, Kim BO. Gait characteristics in down’s syndrome. Gait & Posture. 1995; 3(2):84. [DOI:10.1016/0966-6362(95)93460-T]
- Smith BA, Ulrich BD. Early onset of stabilizing strategies for gait and obstacles: Older adults with down syndrome. Gait & Posture. 2008; 28(3):448-55. [DOI:10.1016/j.gaitpost.2008.02.002] [PMID] [PMCID]
- Gretz HR, Doering LL, Quinn J, Raftopoulos M, Nelson AJ, Zwick DE. Functional ambulation performance testing of adults with down syndrome. NeuroRehabilitation. 1998; 11(3):211-25. [DOI:10.1016/S1053-8135(98)00023-7]
- Vismara L, Romei M, Galli M, Montesano A, Baccalaro G, Crivellini M, et al. Clinical implications of gait analysis in the rehabilitation of adult patients with” Prader-Willi” syndrome: A cross-sectional comparative study (“ Prader-Willi” syndrome vs matched obese patients and healthy subjects). Journal of NeuroEngineering and Rehabilitation. 2007; 4:14. [DOI:10.1186/1743-0003-4-14] [PMID] [PMCID]
- Cimolin V, Galli M, Grugni G, Vismara L, Albertini G, Rigoldi C, et al. Gait patterns in Prader-Willi and down syndrome patients. Journal of NeuroEngineering and Rehabilitation. 2010; 7:28. [DOI:10.1186/1743-0003-7-28] [PMID] [PMCID]
- Rigoldi C, Galli M, Cimolin V, Camerota F, Celletti C, Tenore N, et al. Gait strategy in patients with Ehlers-Danlos syndrome hypermobility type and Down syndrome. Research in Developmental Disabilities. 2012; 33(5):1437-42. [DOI:10.1016/j.ridd.2012.03.016] [PMID]
- Sparrow W, Shinkfield AJ, Summers J. Gait characteristics in individuals with mental retardation: Unobstructed level-walking, negotiating obstacles, and stair climbing. Human Movement Science. 1998; 17(2):167-87. [DOI:10.1016/S0167-9457(97)00028-6]
- Rigoldi C, Galli M, Albertini G. Gait development during lifespan in subjects with down syndrome. Research in Developmental Disabilities. 2011; 32(1):158-63. [DOI:10.1016/j.ridd.2010.09.009] [PMID]
- Galli M, Rigoldi C, Brunner R, Virji Babul N, Giorgio A. Joint stiffness and gait pattern evaluation in children with Down syndrome. Gait & Posture. 2008; 28(3):502-6. [DOI:10.1016/j.gaitpost.2008.03.001] [PMID]
- Rigoldi C, Galli M, Tenore N, Onorati P, Carducci F, Crivellini M, et al. Relation between quantitative motion analysis and cerebral volumes analysis in down syndrome subjects. Gait & Posture. 2008; 29(1):e31. [DOI:10.1016/j.gaitpost.2008.10.050]
- Haynes CA, Lockhart TE. Evaluation of gait and slip parameters for adults with intellectual disability. Journal of Biomechanics. 2012; 45(14):2337-41. [DOI:10.1016/j.jbiomech.2012.07.003] [PMID] [PMCID]
- Cioni M, Cocilovo A, Rossi F, Paci D, Valle MS. Analysis of ankle kinetics during walking in individuals with Down syndrome. American Journal of Mental Retardation. 2001; 106(5):470-8. [DOI:10.1352/0895-8017(2001)1062.0.CO;2]
- Wu J, Ajisafe T. Kinetic patterns of treadmill walking in preadolescents with and without down syndrome. Gait & Posture. 2014; 39(1):241-6. [DOI:10.1016/j.gaitpost.2013.07.113] [PMID]
- Oppewal A, Hilgenkamp TI. The dual task effect on gait in adults with intellectual disabilities: Is it predictive for falls? Disability and Rehabilitation. 2017:1-7. [DOI:10.1080/09638288.2017.1370730] [PMID]
- Agiovlasitis S, McCubbin JA, Yun J, Mpitsos G, Pavol MJ. Effects of down syndrome on three-dimensional motion during walking at different speeds. Gait & Posture. 2009; 30(3):345-50. [DOI:10.1016/j.gaitpost.2009.06.003] [PMID]
- Agiovlasitis S, McCubbin JA, Yun J, Pavol MJ, Widrick JJ. Economy and preferred speed of walking in adults with and without Down syndrome. Adapted Physical Activity Quarterly. 2009; 26(2):118-30. [DOI:10.1123/apaq.26.2.118] [PMID]
- Lopes Pedralli M, Schelle GH. Gait evaluation in individuals with down syndrome. Brazilian Journal of Biomotricity. 2013; 7(1):20-7.
- Looper J, Benjamin D, Nolan M, Schumm L. What to measure when determining orthotic needs in children with Down syndrome: A pilot study. Pediatric Physical Therapy. 2012; 24(4):313-9. [DOI:10.1097/PEP.0b013e31826896eb] [PMID]
- Lee KJ, Lee MM, Shin DC, Shin SH, Song CH. The effects of a balance exercise program for enhancement of gait function on temporal and spatial gait parameters in young people with intellectual disabilities. Journal of Physical Therapy Science. 2014; 26(4):513-6. [DOI:10.1589/jpts.26.513] [PMID] [PMCID]
- Carmeli E, Kessel S, Bar Chad S, Merrick J. A comparison between older persons with down syndrome and a control group: clinical characteristics, functional status and sensorimotor function. Down’s Syndrome, Research and Practice. 2004; 9(1):17-24. [PMID]
- Lahtinen U, Rintala P, Malin A. Physical performance of individuals with intellectual disability: A 30-year follow-up. Adapted Physical Activity Quarterly. 2007; 24(2):125-43. [DOI:10.1123/apaq.24.2.125] [PMID]
- Carvalho R, Almeida G. Assessment of postural adjustments in persons with intellectual disability during balance on the seesaw. Journal of Intellectual Disability Research. 2009; 53(4):389-95. [DOI:10.1111/j.1365-2788.2008.01147.x] [PMID]
- Okuzumi H, Tanaka A, Haishi K. Relationship between age and head movement during stepping in place by nonhandicapped children and persons with mental retardation. Perceptual and Motor Skills. 1997; 85(1):375-81. [DOI:10.2466/pms.1997.85.1.375] [PMID]
- Dellavia C, Pallavera A, Orlando F, Sforza C. Postural stability of athletes in special olympics. Perceptual and Motor Skills. 2009; 108(2):608-22. [DOI:10.2466/pms.108.2.608-622] [PMID]
- Gomes MM, Barela JA. Postural control in Down syndrome: The use of somatosensory and visual information to attenuate body sway. Motor Control. 2007; 11(3):224-34. [DOI:10.1123/mcj.11.3.224]
- Hale L, Miller R, Barach A, Skinner M, Gray A. Motor control test responses to balance perturbations in adults with an intellectual disability. Journal of Intellectual and Developmental Disability. 2009; 34(1):81-6. [DOI:10.1080/13668250802683810] [PMID]
- Blomqvist S, Olsson J, Wallin L, Wester A, Rehn B. Adolescents with intellectual disability have reduced postural balance and muscle performance in trunk and lower limbs compared to peers without intellectual disability. Research in Developmental Disabilities. 2013; 34(1):198-206. [DOI:10.1016/j.ridd.2012.07.008] [PMID]
- Kokubun M, Shinmyo T, Ogita M, Morita K, Furuta M, Haishi K, et al. Comparison of postural control of children with down syndrome and those with other forms of mental retardation. Perceptual and Motor Skills. 1997; 84(2):499-504. [DOI:10.2466/pms.1997.84.2.499] [PMID]
- Hale L, Bray A, Littmann A. Assessing the balance capabilities of people with profound intellectual disabilities who have experienced a fall. Journal of Intellectual Disability Research. 2007; 51(4):260-8. [DOI:10.1111/j.1365-2788.2006.00873.x] [PMID]
- Webber A, Virji Babul N, Edwards R, Lesperance M. Stiffness and postural stability in adults with down syndrome. Experimental Brain Research. 2004; 155(4):450-8. [DOI:10.1007/s00221-003-1743-7] [PMID]
- Suomi R, Koceja DM. Postural sway patterns of normal men and women and men with mental retardation during a two-legged stance test. Archives of Physical Medicine and Rehabilitation. 1994; 75(2):205-9. [PMID]
- DePaepe JL, Ciccaglione S. A dynamic balance measure for persons with severe and profound mental retardation. Perceptual and Motor Skills. 1993; 76(2):619-27. [DOI:10.2466/pms.1993.76.2.619] [PMID]
- Van Emmerik R, Sprague R, Newell K. Quantification of postural sway patterns in tardive dyskinesia. Movement Disorders. 1993; 8(3):305-14. [DOI:10.1002/mds.870080309] [PMID]
- Ko Y, Emmerik R, Sprague R, Newell K. Postural stability, tardive dyskinesia and developmental disability. Journal of Intellectual Disability Research. 1992; 36(4):309-23. [DOI:10.1111/j.1365-2788.1992.tb00530.x] [PMID]
- Bahiraei S, Daneshmandi H. [The study of relationship between structural profiles and postural control in individual with down syndrome (Persian)]. Journal of Practical Studies of Biosciences in Sports. 2014; 2(4):21-32.
- Pitetti K, Miller RA, Loovis M. Balance and coordination capacities of male children and adolescents with intellectual disability. Adapted Physical Activity Quarterly. 2017; 34(1):1-18. [DOI:10.1123/APAQ.2016-0010] [PMID]
- Watson N, Rosano C, Boudreau R, Simonsick E, Ferrucci L, Sutton-Tyrrell K, et al. Executive function, memory, and gait speed decline in well-functioning older adults. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2010; 65(10):1093-100. [DOI:10.1093/gerona/glq111] [PMID] [PMCID]
- Montero‐Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society. 2012; 60(11):2127-36. [DOI:10.1111/j.1532-5415.2012.04209.x] [PMID] [PMCID]
- Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: Evidence and implications. Movement Disorders. 2013; 28(11):1520-33. [DOI:10.1002/mds.25674] [PMID] [PMCID]
- Axer H, Axer M, Sauer H, Witte OW, Hagemann G. Falls and gait disorders in geriatric neurology. Clinical Neurology and Neurosurgery. 2010; 112(4):265-74. [DOI:10.1016/j.clineuro.2009.12.015] [PMID]
- Chambers HG, Sutherland DH. A practical guide to gait analysis. Journal of the American Academy of Orthopaedic Surgeons. 2002; 10(3):222-31. [DOI:10.5435/00124635-200205000-00009]
- Wert DM, Brach J, Perera S, VanSwearingen JM. Gait biomechanics, spatial and temporal characteristics, and the energy cost of walking in older adults with impaired mobility. Physical Therapy. 2010; 90(7):977-85. [DOI:10.2522/ptj.20090316] [PMID] [PMCID]
- Verlinden VJ, van der Geest JN, Hofman A, Ikram MA. Cognition and gait show a distinct pattern of association in the general population. Alzheimer’s & Dementia. 2014; 10(3):328-35. [DOI:10.1016/j.jalz.2013.03.009] [PMID]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011; 305(1):50-8. [DOI:10.1001/jama.2010.1923] [PMID] [PMCID]
- Finlayson J, Morrison J, Jackson A, Mantry D, Cooper SA. Injuries, falls and accidents among adults with intellectual disabilities. Prospective cohort study. Journal of Intellectual Disability Research. 2010; 54(11):966-80. [DOI:10.1111/j.1365-2788.2010.01319.x] [PMID]
Type of Study: Research |
Subject:
Sport injury and corrective exercises
Received: 2018/01/10 | Accepted: 2018/05/15 | Published: 2018/07/1
Received: 2018/01/10 | Accepted: 2018/05/15 | Published: 2018/07/1
References
1. Harris JC. Intellectual disability: Understanding its development, causes, classification, evaluation, and treatment. Oxford: Oxford University Press; 2006.
2. Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: A meta-analysis of population-based studies. Research in Developmental Disabilities. 2011; 32(2):419-36. [DOI:10.1016/j.ridd.2010.12.018] [PMID] [DOI:10.1016/j.ridd.2010.12.018]
3. Wu J, Ulrich DA, Looper J, Tiernan CW, Angulo Barroso RM. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with Down syndrome after different treadmill interventions. Experimental Brain Research. 2008; 186(2):261-72. [DOI:10.1007/s00221-007-1230-7] [PMID] [DOI:10.1007/s00221-007-1230-7]
4. Rahmani P, Shahrokhi H. The study of static and dynamic balance in mentally retarded female students with and without Down Syndrome (DS). Journal of Sports Medicine. 2012; 2(2):97-113.
5. Cabeza Ruiz R, García Massó X, Centeno Prada R, Beas Jiménez J, Colado J, González LM. Time and frequency analysis of the static balance in young adults with Down syndrome. Gait & Posture. 2011; 33(1):23-8. [DOI:10.1016/j.gaitpost.2010.09.014] [PMID] [DOI:10.1016/j.gaitpost.2010.09.014]
6. Galli M, Rigoldi C, Mainardi L, Tenore N, Onorati P, Albertini G. Postural control in patients with Down syndrome. Disability and Rehabilitation. 2008; 30(17):1274-8. [DOI:10.1080/09638280701610353] [PMID] [DOI:10.1080/09638280701610353]
7. World Health Organization. nternational Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2007.
8. Evenhuis HM. Want ik wil nog lang leven: Moderne gezondheidszorg voor mensen met verstandelijke beperkingen: Zoetermeer: Raad voor de Volksgezondheid en Zorg; 2002.
9. Janicki M, Dalton AJ, Michael Henderson C, Davidson PW. Mortality and morbidity among older adults with intellectual disability: Health services considerations. Disability and Rehabilitation. 1999; 21(5-6):284-94. [DOI:10.1080/096382899297710] [PMID] [DOI:10.1080/096382899297710]
10. Hall JM, Thomas MJ. Promoting physical activity and exercise in older adults with developmental disabilities. Topics in Geriatric Rehabilitation. 2008; 24(1):64-73. [DOI:10.1097/01.TGR.0000311407.09178.55] [DOI:10.1097/01.TGR.0000311407.09178.55]
11. Horvat M, Croce R, Zagrodnik J, Brooks B, Carter K. Spatial and temporal variability of movement parameters in individuals with down syndrome. Perceptual and Motor Skills. 2012; 114(3):774-82. [DOI:10.2466/25.15.26.PMS.114.3.774-782] [PMID] [DOI:10.2466/25.15.26.PMS.114.3.774-782]
12. Kim BS, Bang D, Kim BO. Gait characteristics in down's syndrome. Gait & Posture. 1995; 3(2):84. [DOI:10.1016/0966-6362(95)93460-T] [DOI:10.1016/0966-6362(95)93460-T]
13. Smith BA, Ulrich BD. Early onset of stabilizing strategies for gait and obstacles: Older adults with down syndrome. Gait & Posture. 2008; 28(3):448-55. [DOI:10.1016/j.gaitpost.2008.02.002] [PMID] [PMCID] [DOI:10.1016/j.gaitpost.2008.02.002]
14. Gretz HR, Doering LL, Quinn J, Raftopoulos M, Nelson AJ, Zwick DE. Functional ambulation performance testing of adults with down syndrome. NeuroRehabilitation. 1998; 11(3):211-25. [DOI:10.1016/S1053-8135(98)00023-7] [DOI:10.1016/S1053-8135(98)00023-7]
15. Vismara L, Romei M, Galli M, Montesano A, Baccalaro G, Crivellini M, et al. Clinical implications of gait analysis in the rehabilitation of adult patients with" Prader-Willi" syndrome: A cross-sectional comparative study (" Prader-Willi" syndrome vs matched obese patients and healthy subjects). Journal of NeuroEngineering and Rehabilitation. 2007; 4:14. [DOI:10.1186/1743-0003-4-14] [PMID] [PMCID] [DOI:10.1186/1743-0003-4-14]
16. Cimolin V, Galli M, Grugni G, Vismara L, Albertini G, Rigoldi C, et al. Gait patterns in Prader-Willi and down syndrome patients. Journal of NeuroEngineering and Rehabilitation. 2010; 7:28. [DOI:10.1186/1743-0003-7-28] [PMID] [PMCID] [DOI:10.1186/1743-0003-7-28]
17. Rigoldi C, Galli M, Cimolin V, Camerota F, Celletti C, Tenore N, et al. Gait strategy in patients with Ehlers-Danlos syndrome hypermobility type and Down syndrome. Research in Developmental Disabilities. 2012; 33(5):1437-42. [DOI:10.1016/j.ridd.2012.03.016] [PMID] [DOI:10.1016/j.ridd.2012.03.016]
18. Sparrow W, Shinkfield AJ, Summers J. Gait characteristics in individuals with mental retardation: Unobstructed level-walking, negotiating obstacles, and stair climbing. Human Movement Science. 1998; 17(2):167-87. [DOI:10.1016/S0167-9457(97)00028-6] [DOI:10.1016/S0167-9457(97)00028-6]
19. Rigoldi C, Galli M, Albertini G. Gait development during lifespan in subjects with down syndrome. Research in Developmental Disabilities. 2011; 32(1):158-63. [DOI:10.1016/j.ridd.2010.09.009] [PMID] [DOI:10.1016/j.ridd.2010.09.009]
20. Galli M, Rigoldi C, Brunner R, Virji Babul N, Giorgio A. Joint stiffness and gait pattern evaluation in children with Down syndrome. Gait & Posture. 2008; 28(3):502-6. [DOI:10.1016/j.gaitpost.2008.03.001] [PMID] [DOI:10.1016/j.gaitpost.2008.03.001]
21. Rigoldi C, Galli M, Tenore N, Onorati P, Carducci F, Crivellini M, et al. Relation between quantitative motion analysis and cerebral volumes analysis in down syndrome subjects. Gait & Posture. 2008; 29(1):e31. [DOI:10.1016/j.gaitpost.2008.10.050] [DOI:10.1016/j.gaitpost.2008.10.050]
22. Haynes CA, Lockhart TE. Evaluation of gait and slip parameters for adults with intellectual disability. Journal of Biomechanics. 2012; 45(14):2337-41. [DOI:10.1016/j.jbiomech.2012.07.003] [PMID] [PMCID] [DOI:10.1016/j.jbiomech.2012.07.003]
23. Cioni M, Cocilovo A, Rossi F, Paci D, Valle MS. Analysis of ankle kinetics during walking in individuals with Down syndrome. American Journal of Mental Retardation. 2001; 106(5):470-8. [DOI:10.1352/0895-8017(2001)1062.0.CO;2]
https://doi.org/10.1352/0895-8017(2001)106<0470:AOAKDW>2.0.CO;2 [DOI:10.1352/0895-8017(2001)1062.0.CO;2]
24. Wu J, Ajisafe T. Kinetic patterns of treadmill walking in preadolescents with and without down syndrome. Gait & Posture. 2014; 39(1):241-6. [DOI:10.1016/j.gaitpost.2013.07.113] [PMID] [DOI:10.1016/j.gaitpost.2013.07.113]
25. Oppewal A, Hilgenkamp TI. The dual task effect on gait in adults with intellectual disabilities: Is it predictive for falls? Disability and Rehabilitation. 2017:1-7. [DOI:10.1080/09638288.2017.1370730] [PMID] [DOI:10.1080/09638288.2017.1370730]
26. Agiovlasitis S, McCubbin JA, Yun J, Mpitsos G, Pavol MJ. Effects of down syndrome on three-dimensional motion during walking at different speeds. Gait & Posture. 2009; 30(3):345-50. [DOI:10.1016/j.gaitpost.2009.06.003] [PMID] [DOI:10.1016/j.gaitpost.2009.06.003]
27. Agiovlasitis S, McCubbin JA, Yun J, Pavol MJ, Widrick JJ. Economy and preferred speed of walking in adults with and without Down syndrome. Adapted Physical Activity Quarterly. 2009; 26(2):118-30. [DOI:10.1123/apaq.26.2.118] [PMID] [DOI:10.1123/apaq.26.2.118]
28. Lopes Pedralli M, Schelle GH. Gait evaluation in individuals with down syndrome. Brazilian Journal of Biomotricity. 2013; 7(1):20-7.
29. Looper J, Benjamin D, Nolan M, Schumm L. What to measure when determining orthotic needs in children with Down syndrome: A pilot study. Pediatric Physical Therapy. 2012; 24(4):313-9. [DOI:10.1097/PEP.0b013e31826896eb] [PMID] [DOI:10.1097/PEP.0b013e31826896eb]
30. Lee KJ, Lee MM, Shin DC, Shin SH, Song CH. The effects of a balance exercise program for enhancement of gait function on temporal and spatial gait parameters in young people with intellectual disabilities. Journal of Physical Therapy Science. 2014; 26(4):513-6. [DOI:10.1589/jpts.26.513] [PMID] [PMCID] [DOI:10.1589/jpts.26.513]
31. Carmeli E, Kessel S, Bar Chad S, Merrick J. A comparison between older persons with down syndrome and a control group: clinical characteristics, functional status and sensorimotor function. Down's Syndrome, Research and Practice. 2004; 9(1):17-24. [PMID] [PMID]
32. Lahtinen U, Rintala P, Malin A. Physical performance of individuals with intellectual disability: A 30-year follow-up. Adapted Physical Activity Quarterly. 2007; 24(2):125-43. [DOI:10.1123/apaq.24.2.125] [PMID] [DOI:10.1123/apaq.24.2.125]
33. Carvalho R, Almeida G. Assessment of postural adjustments in persons with intellectual disability during balance on the seesaw. Journal of Intellectual Disability Research. 2009; 53(4):389-95. [DOI:10.1111/j.1365-2788.2008.01147.x] [PMID] [DOI:10.1111/j.1365-2788.2008.01147.x]
34. Okuzumi H, Tanaka A, Haishi K. Relationship between age and head movement during stepping in place by nonhandicapped children and persons with mental retardation. Perceptual and Motor Skills. 1997; 85(1):375-81. [DOI:10.2466/pms.1997.85.1.375] [PMID] [DOI:10.2466/pms.1997.85.1.375]
35. Dellavia C, Pallavera A, Orlando F, Sforza C. Postural stability of athletes in special olympics. Perceptual and Motor Skills. 2009; 108(2):608-22. [DOI:10.2466/pms.108.2.608-622] [PMID] [DOI:10.2466/pms.108.2.608-622]
36. Gomes MM, Barela JA. Postural control in Down syndrome: The use of somatosensory and visual information to attenuate body sway. Motor Control. 2007; 11(3):224-34. [DOI:10.1123/mcj.11.3.224] [DOI:10.1123/mcj.11.3.224]
37. Hale L, Miller R, Barach A, Skinner M, Gray A. Motor control test responses to balance perturbations in adults with an intellectual disability. Journal of Intellectual and Developmental Disability. 2009; 34(1):81-6. [DOI:10.1080/13668250802683810] [PMID] [DOI:10.1080/13668250802683810]
38. Blomqvist S, Olsson J, Wallin L, Wester A, Rehn B. Adolescents with intellectual disability have reduced postural balance and muscle performance in trunk and lower limbs compared to peers without intellectual disability. Research in Developmental Disabilities. 2013; 34(1):198-206. [DOI:10.1016/j.ridd.2012.07.008] [PMID] [DOI:10.1016/j.ridd.2012.07.008]
39. Kokubun M, Shinmyo T, Ogita M, Morita K, Furuta M, Haishi K, et al. Comparison of postural control of children with down syndrome and those with other forms of mental retardation. Perceptual and Motor Skills. 1997; 84(2):499-504. [DOI:10.2466/pms.1997.84.2.499] [PMID] [DOI:10.2466/pms.1997.84.2.499]
40. Hale L, Bray A, Littmann A. Assessing the balance capabilities of people with profound intellectual disabilities who have experienced a fall. Journal of Intellectual Disability Research. 2007; 51(4):260-8. [DOI:10.1111/j.1365-2788.2006.00873.x] [PMID] [DOI:10.1111/j.1365-2788.2006.00873.x]
41. Webber A, Virji Babul N, Edwards R, Lesperance M. Stiffness and postural stability in adults with down syndrome. Experimental Brain Research. 2004; 155(4):450-8. [DOI:10.1007/s00221-003-1743-7] [PMID] [DOI:10.1007/s00221-003-1743-7]
42. Suomi R, Koceja DM. Postural sway patterns of normal men and women and men with mental retardation during a two-legged stance test. Archives of Physical Medicine and Rehabilitation. 1994; 75(2):205-9. [PMID] [PMID]
43. DePaepe JL, Ciccaglione S. A dynamic balance measure for persons with severe and profound mental retardation. Perceptual and Motor Skills. 1993; 76(2):619-27. [DOI:10.2466/pms.1993.76.2.619] [PMID] [DOI:10.2466/pms.1993.76.2.619]
44. Van Emmerik R, Sprague R, Newell K. Quantification of postural sway patterns in tardive dyskinesia. Movement Disorders. 1993; 8(3):305-14. [DOI:10.1002/mds.870080309] [PMID] [DOI:10.1002/mds.870080309]
45. Ko Y, Emmerik R, Sprague R, Newell K. Postural stability, tardive dyskinesia and developmental disability. Journal of Intellectual Disability Research. 1992; 36(4):309-23. [DOI:10.1111/j.1365-2788.1992.tb00530.x] [PMID] [DOI:10.1111/j.1365-2788.1992.tb00530.x]
46. Bahiraei S, Daneshmandi H. [The study of relationship between structural profiles and postural control in individual with down syndrome (Persian)]. Journal of Practical Studies of Biosciences in Sports. 2014; 2(4):21-32.
47. Pitetti K, Miller RA, Loovis M. Balance and coordination capacities of male children and adolescents with intellectual disability. Adapted Physical Activity Quarterly. 2017; 34(1):1-18. [DOI:10.1123/APAQ.2016-0010] [PMID] [DOI:10.1123/APAQ.2016-0010]
48. Watson N, Rosano C, Boudreau R, Simonsick E, Ferrucci L, Sutton-Tyrrell K, et al. Executive function, memory, and gait speed decline in well-functioning older adults. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2010; 65(10):1093-100. [DOI:10.1093/gerona/glq111] [PMID] [PMCID] [DOI:10.1093/gerona/glq111]
49. Montero‐Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society. 2012; 60(11):2127-36. [DOI:10.1111/j.1532-5415.2012.04209.x] [PMID] [PMCID] [DOI:10.1111/j.1532-5415.2012.04209.x]
50. Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: Evidence and implications. Movement Disorders. 2013; 28(11):1520-33. [DOI:10.1002/mds.25674] [PMID] [PMCID] [DOI:10.1002/mds.25674]
51. Axer H, Axer M, Sauer H, Witte OW, Hagemann G. Falls and gait disorders in geriatric neurology. Clinical Neurology and Neurosurgery. 2010; 112(4):265-74. [DOI:10.1016/j.clineuro.2009.12.015] [PMID] [DOI:10.1016/j.clineuro.2009.12.015]
52. Chambers HG, Sutherland DH. A practical guide to gait analysis. Journal of the American Academy of Orthopaedic Surgeons. 2002; 10(3):222-31. [DOI:10.5435/00124635-200205000-00009] [DOI:10.5435/00124635-200205000-00009]
53. Wert DM, Brach J, Perera S, VanSwearingen JM. Gait biomechanics, spatial and temporal characteristics, and the energy cost of walking in older adults with impaired mobility. Physical Therapy. 2010; 90(7):977-85. [DOI:10.2522/ptj.20090316] [PMID] [PMCID] [DOI:10.2522/ptj.20090316]
54. Verlinden VJ, van der Geest JN, Hofman A, Ikram MA. Cognition and gait show a distinct pattern of association in the general population. Alzheimer's & Dementia. 2014; 10(3):328-35. [DOI:10.1016/j.jalz.2013.03.009] [PMID] [DOI:10.1016/j.jalz.2013.03.009]
55. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011; 305(1):50-8. [DOI:10.1001/jama.2010.1923] [PMID] [PMCID] [DOI:10.1001/jama.2010.1923]
56. Finlayson J, Morrison J, Jackson A, Mantry D, Cooper SA. Injuries, falls and accidents among adults with intellectual disabilities. Prospective cohort study. Journal of Intellectual Disability Research. 2010; 54(11):966-80. [DOI:10.1111/j.1365-2788.2010.01319.x] [PMID] [DOI:10.1111/j.1365-2788.2010.01319.x]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |