Sat, Jan 31, 2026
Volume 14, Issue 3 (Summer 2024)
PTJ 2024, 14(3): 171-182 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Charehjou B, Moghadas Tabrizi Y, Minoonejad H. The Combination of Transcranial Direct Current Stimulation and Virtual Reality Training on Fatigue, Balance and Walking in Patients With Multiple Sclerosis. PTJ 2024; 14 (3) :171-182
URL: http://ptj.uswr.ac.ir/article-1-593-en.html
URL: http://ptj.uswr.ac.ir/article-1-593-en.html
1- Department of Sports Injuries and Biomechanics, School of Sport Sciences and Health, University of Tehran, Tehran, Iran.
Keywords: Multiple sclerosis (MS), Virtual reality (VR), Transcranial direct current stimulation (tDCS), Combined effect
Full-Text [PDF 701 kb]
(1625 Downloads)
| Abstract (HTML) (3936 Views)
Full-Text: (1181 Views)
Introduction
Multiple sclerosis (MS) is a chronic and degenerative neurological disease, with myelin sheath damage in the central nervous system (CNS) [1]. Pathological features of the disease, such as inflammation, demyelination, and destruction of neurons differently appear [2]. The most common non-traumatic CNS injury in young adults with an average age of 20-40 years is MS [3]. The clinical course of the disease is heterogeneous, including mild neurological symptoms to progressive and debilitating forms [3]. Due to the demyelination, patients experience simple or multiple sensory, motor, emotional-behavioral, and cognitive symptoms [4], depending on the location of the lesions [5]. Common symptoms of the disease include muscle weakness, spasticity, fatigue, sensory dysfunctions, balance problems, cognitive disorders, and difficulty in walking [6] Symptoms significantly affect a patient’s social communication, quality of life, job, and performance [7].
Fatigue, as one of the common and debilitating symptoms [8], affects about 80% of patients at different stages of the disease [9]. It reduces cognitive and functional abilities, associated with increased need for rest and decreased motivation [10]. Primary and secondary progressive stages of the disease showed more fatigue symptoms compared to relapsing-remitting MS [4]. The pathophysiology of fatigue in MS is not known [11], but it occurs as a result of brain dysfunction (primary fatigue) or following symptoms during the disease (secondary fatigue) [12]. Primary fatigue results from the pathophysiological process of the disease itself, and also secondary fatigue is caused by the complications of the disease, such as endocrine disorders, infections, lack of vitamins, and anemia [8, 10]. People with MS can have limitations in daily activities due to muscle weakness, spasticity, and imbalance. Therefore, it has a bad effect on walking and increases the risk of falling. More than spasticity in MS people, it prevents functional activities, such as moving, which increases disability. Therefore, fatigue has a negative effective (mentally and physically) life and the ability to work in patients with MS by limiting daily activities and coping abilities [13, 14].
Imbalance is one of the primary symptoms of MS associated with an increased risk of falling [15, 16]. Reduced functional capacity, muscle weakness, fatigue, and spasms, which are common symptoms in these patients, may lead to inappropriate balance [17, 18]. This debilitating symptom has been reported in 75% of patients, which can reduce the mobility and independence of the affected person and ultimately affect his/her quality of life [19]. Balance problems occur at the beginning of the disease and usually increase with the progress of the disease [20].
Walking disorder is also commonly seen in MS patients and is described as the most challenging symptom by 70% of patients [21, 22]. The patterns of walking disorders that are reflected as asymmetry and coordination dysfunction in patients reduce walking speed and increase energy consumption [23]. Walking disorders appear in different ways, including decreasing the length of the step, reducing the speed, and increasing the width of the step [24]. Walking and balance problems lead to an increase in falling risk, activity limitation, and isolation, which are worsened by the progression of the disease [25].
Pharmacological treatment controls the symptoms and consequences of the disease, but the results are not clinically satisfactory. Alternative therapies have introduced to relieve existing symptoms and to prevent complications of MS [26].
Exercise training and behavioral therapy are both useful for patients with mild disease symptoms [1]. In recent years, in addition to physical exercises, the number of studies on the effectiveness of other non-drug methods, including non-invasive brain stimulation techniques, has increased. One of these effective methods is transcranial direct current electrical stimulation (tDCS), which is easy to use, and is considered a cheap and non-invasive tool for the motor rehabilitation of patients [6, 25]. tDCS produces a low-amplitude direct current that can change cortical excitability without harmful side effects [2, 8]. Stimulation with anodic direct current increases the resting potential of the neuronal membrane, while with cathode current resting potential [26].
tDCS is widely used in the rehabilitation of various neurological diseases, such as stroke, Parkinson’s disease, and MS [27]. Navarra-Lopez et al. in their systematic review reported that tDCS along with physical therapy can improve the walking parameters, static and dynamic balance, and lower limb function in stroke [28]. Another study conducted on people with Parkinson’s disease in 2020 showed that one session of bilateral and electrical stimulation of the cerebellum can significantly improve balance performance [29].
Among other techniques in rehabilitation, virtual reality (VR), focusing on neural augmentation and motor learning is an effective method that can be used as an alternative to traditional rehabilitation in MS patients [1]. VR provides the user with an alternative and favorable simulation of activity or environment that permits interaction through multiple sensory systems [30]. The environment contains various stimulation which produces a potent signal to reorganize sensorimotor circuits which can affect the motor cortex during motor learning [31]. By the way, repeated and purposeful observation of activities can influence cortico-cortical interactions in the premotor and motor areas [32]. Therefore VR prepares relevant and meaningful stimulation to individual’s different brain areas, promoting motor learning and rehabilitation via neuroplasticity. VR not only improves patients’ quality of life but also has a role in the return of their brain health [33]. In recent years, VR technology has increasingly become affordable, flexible, and portable, which enables researchers to consider its use in many fields, especially the medical field [34, 35]. In a systematic study and meta-analysis, Zhang et al. confirmed that VR training is effective in the motor function of the upper and lower limbs, walking, balance, and daily activity of stroke patients and improves variables, but without effect on cognition [36]. Moreover, the study conducted by Abou L et al showed positive effects of VR training that improves balance during sitting and standing and a trend of gait improvement in persons with SCI [37]. Another study performed by Wang et al. [38] on the effectiveness of VR on the balance and walking of people with Parkinson’s showed a significant effect on balance with no effect on walking [38].
The limited studies evaluated the effects of tDCS and VR on complications, such as fatigue, balance disorder, and walking in people with MS. Therefore, the present study was conducted to evaluate the effect of tDCS and VR on the mentioned symptoms of the disease. In addition, no investigation was found to study the combined effects of VR and tDCS on the mentioned variables. The synergistic effects of these two intervention methods can lead to greater effectiveness and shorter treatment time. Therefore, the secondary goal of this investigation was to compare the therapeutic effects of VR and tDCS separately and in combination on the level of fatigue, balance, and some walking parameters (speed & stride length) of people with MS.
Materials and Methods
The participants included 30 patients with MS aged 18-55 years. Patients whose disease was confirmed by neurologists participated in the present study voluntarily and purposefully. The inclusion criteria included a patient with expanded disability status scale (EDSS) ≤6 (EDSS is used to quantify disability and monitor its alterations during the time in MS) and not having any type of severe visual impairment, no history of concussion, ability to walk independently with or without an assistive device and not having any attack during last month. By the way, the exclusion criteria included patients with any severe systemic disorder, such as epilepsy or psychiatric disease, which prevented the use of VR modality and electrical stimulation. Participants entered the study after completing the written informed consent form and had the option to withdraw from the research at any time. The patients were brain type of MS with their routine MS drug therapy without participating in other rehabilitation interventions. Table 1 presents the demographic information of the subjects.

The present study is a clinical trial study, with pre and post-assessment. The subjects were randomly allocated (by choosing a number from the box) to one of three groups according to the study inclusion/exclusion criteria, tDCS-VR group (n=10), VR group (n=10), and tDCS group (n=10).
The pre-test performed before the intervention included the fatigue severity scale (FSS), modified fatigue effect scale (MFIS), balance sheet scale (BBS), and 25-foot walk test (T25-FW). Immediately after the intervention, these evaluations were repeated as post-test. The intervention stages are shown in Figure 1. The researcher administering all tests was blinded to the test condition.

tDCS
A device (ActivaDose) was used to administer tDCS by a trained therapist. At first, we placed the anode and cathode electrodes in 5×5 cm sponge pads, and the pads were dipped in salt solution. We placed the anode electrode on the motor cortex M1 (C3) on the left side and the cathode on the right forehead according to the international 10-20 system [38]. Then, the stimulation protocol [38] consisting of electrical stimulation of the brain with a direct current of 2 mA, 20 minutes daily for 5 consecutive days was used. As a sham intervention, the current was turned off after 60 s of the onset of stimulation.
VR
The VR protocol was implemented using the VR BOX headset and based on the defined protocol [17, 39] and Costa (2020) [40]. Patients performed the VR program three sessions per week for two weeks. The duration of each session was 20 minutes.
We used the VR BOX headset to implement the VR protocol. In this way, we first put the Android phone inside the headset compartment and then put it on the patient’s face.
The combined protocol was as follows: We placed the anode and cathode electrodes on the M1 motor cortex and on the forehead, respectively. We applied direct electrical stimulation of the brain for two weeks and three seasons every week with a current intensive of 2MA and 20 minutes. After fixing the electrodes in the desired place using a strap, we placed the VR headset on the patient’s face and applied two stimuli simultaneously. VR was also performed for six sessions, three times a week, and for 20 minutes.
The games include Slalom ski, penguin slide, and marble balance:
Slalom ski: This game requires the patient to pass between two flags placed along the track.
Penguin slide: In this game, patients must move from the ice platform to the fishing points outside the platform without falling.
Marble balance: In this game, the patient has to put the ball in a hole. Accordingly, he/she should shift his/her body weight to the direction where he/she wants the ball to be placed. If successful, the patient is transferred to the next stage, which is more difficult.
It should be noted that the VR group was also considered as a sham group and thus therefore simultaneously received sham stimulation by electrodes located according to the mentioned protocol.
During the combined VR+tDCS protocol, the anode and cathode electrodes were placed on the M1 motor cortex and the forehead, respectively, and at the same time, the VR headset was placed on a patient’s face, and both types of intervention were performed simultaneously (tDCS with intensity of 2 mA that simultaneously applied using VR for 20 minutes for two weeks and three sessions each per week).
Tools
FSS: FSS is a 9-item scale that mainly focuses on the physical aspect of fatigue. Each item has a 7-point Likert scale, ranging from 1 (completely disagree) to 7 (completely agree). Therefore, the total score can be from 9 to 63. Having a FSS <4, 4> FSS <5, and FSS >5 is classified as mild, moderate, and severe fatigue, respectively. In the Iranian version of the instrument, Cronbach’s α coefficient and intra-class correlation coefficient (ICC) were reported as 0.96, and 0.93 respectively. A validity of 85% was shown in the researchers’ results [40].
MFIS: MFIS is a 21-item scale that assesses cognitive and psychosocial in addition to physical dimensions with a 5-point Likert scale, ranging from zero (never) to four (almost always). The range of total score is from 0 to 84. A correlation of r=68%, P<0.0001 was reported between the two tests of fatigue intensity scale and modified fatigue effect [41]. A high Cronbach’s score and ICC were reported in the Persian version of MFIS [41].
Berg balance scale (BBS): BBS contains 14 common functional activities that are performed repeatedly in a day in a 4-point Likert scale, ranging from 0 to 4 (0 indicating the lowest level of performance and 4 indicating the highest level of performance). Persian version of the test showed very high inter-rater and test re-test reliability (ICC=0.93 and 0.95, respectively) in the elderly [42, 43].
T 25-F W: T 25-F W as one of the three components of patients with MS function evaluates walking speed. Walking speed in meters per second is obtained by dividing the measured distance of 25 feet by the time. The reliability and validity of the T25-FW is reported as 97% [44].
Statistical analysis
The first normality of data was checked. The dependent t-test was used to compare within-group variables. Then analysis of covariance (ANCOVA) was employed to determine differences between the post-test scores of three groups, using baseline values as covariates while Turkey’s method was used as a post-test. P<0.05 was regarded as statistically significant. SPSS software, version 19, was used for statistical analysis.
Results
All participants completed all stages, and also no drop was observed in the number of samples in the present study. Table 1 presents the demographic characteristics and disability score (Mean±SD) of the groups. Descriptive finding and comparison of within-group assessments were shown in Table 2.
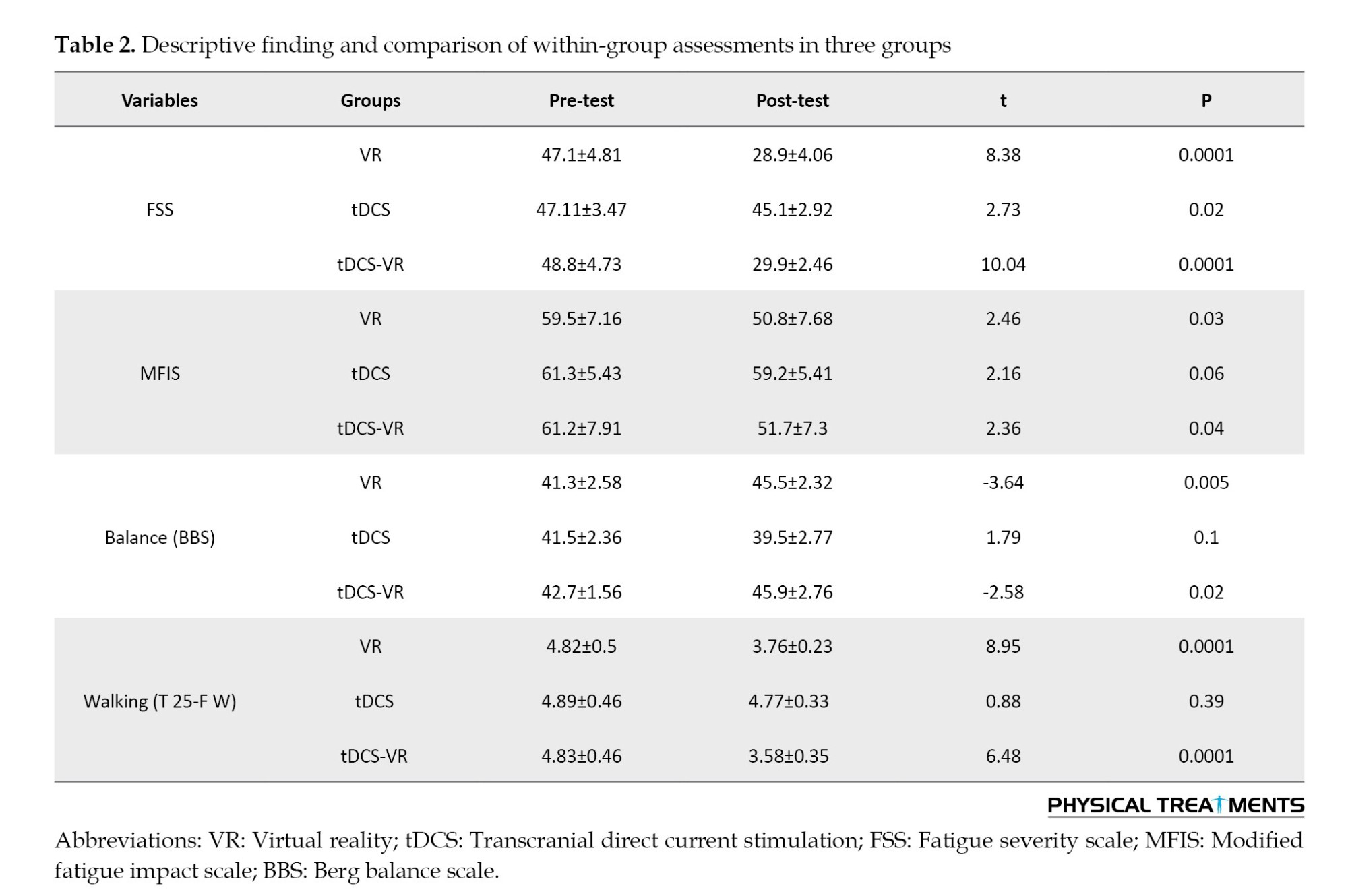
The one-way analysis of covariance (ANCOVA) was used to compare the adjusted mean of the dependent variables in the 2×3 design (three groups in two stages). A significant difference was observed between the post-test-adjusted mean of all dependent variables (P<0.05) (Table 3).
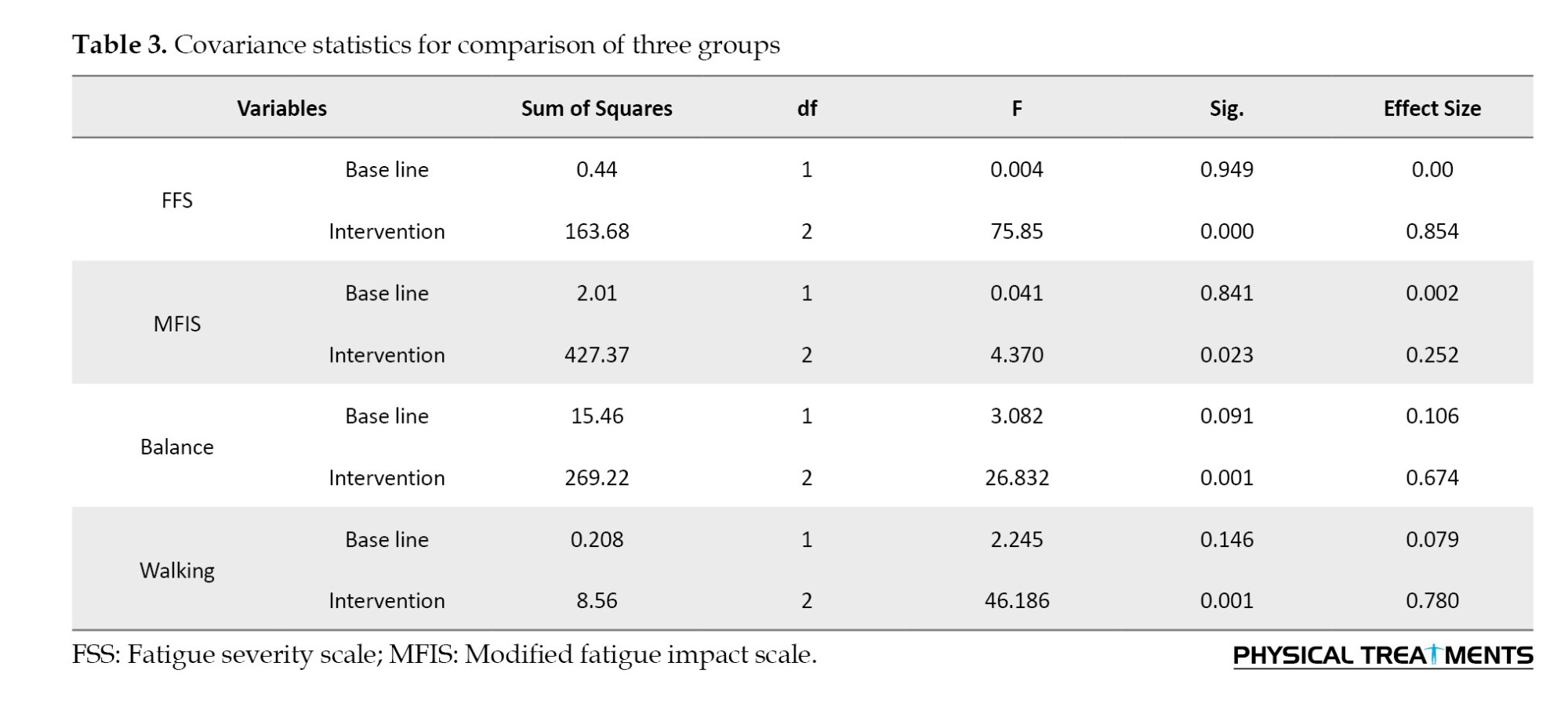
According to Turkey’s post hoc test results of the mean intensity of fatigue, both the VR and the tDCS-VR group significantly had lower scores than the tDCS group (P<0.001); however, the mean fatigue intensity between the VR and the tDCS-VR group was not significant (P=0.990) (Table 4).
Regarding the post hoc results of the balance test, the VR and tDCS-VR groups were significantly better than the tDCS group (P<0.001). No significant difference was observed between the mean balance of the VR group and the tDCS-VR group (P=0.995) (Table 4).
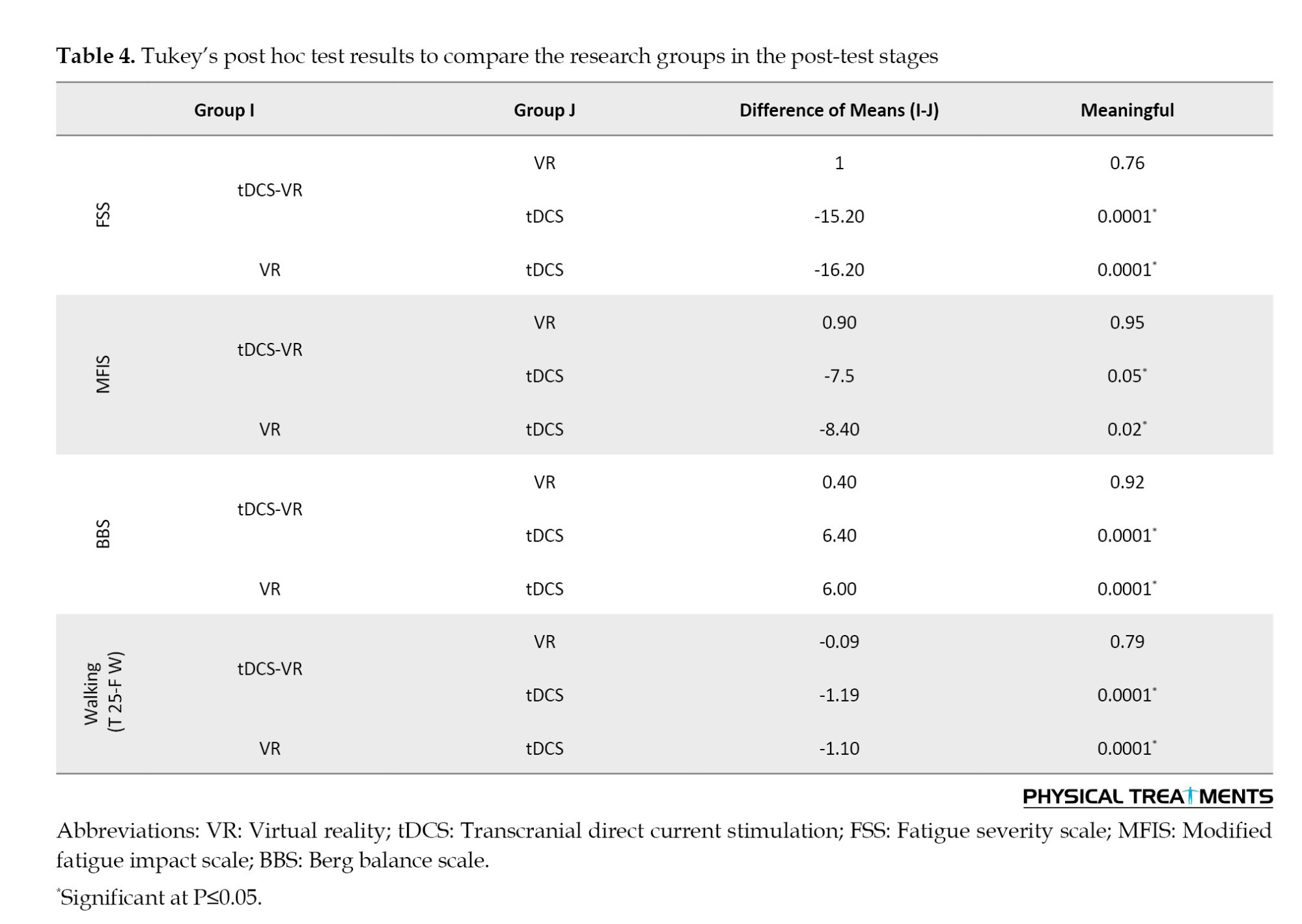
According to the mean walking speed, the VR and the tDCS-VR groups were significantly better than the tDCS group (P<0.001). While the difference between the mean walking speed of the VR and the tDCS-VR groups was not significant (P=1.000).
Moreover, in the mean modified fatigue, the VR group significantly showed lower scores than the tDCS and tDCS-VR groups (P=0.037), therefore it can be said that the VR intervention significantly reduced the modified fatigue compared to the other two interventions.
Discussion
The present study was conducted to investigate the effects of VR and tDCS separately and in combination on fatigue; balance and walking speed of MS patients. The results showed a significant effect of tDCS, VR, and tDCS-VR on fatigue and a significant effect of VR and tDCS-VR on balance and walking speed in patients with MS. In comparing the effect of different interventions on fatigue, balance, and walking speed, the VR and tDCS-VR groups significantly showed better results, but no significant difference was observed between VR and tDCS-VR groups. A significant difference of the modified fatigue was observed between the VR with the other groups.
Fatigue: Our study showed that all three groups (including AtDCS, VR, and tDCS-VR) had a significant decrease in the intensity of fatigue in the post-test compared to the pre-test. The participants in the tDCS group improved severe fatigue symptoms and 8 of them improved the modified fatigue. In the present study, anodic stimulation was used by placing the stimulating electrode on the left M1 region and the cathode on the right frontal region. In previous studies, different areas have been used to reduce fatigue, such as [45] dorsolateral prefrontal cortex (DLPFC), parietal P4 [46] and S1 [47] and M1 [48]. Anodic stimulation can improve fatigue caused by MS by several mechanisms, including the antidepressant effects of AtDCS [49], and the neurochemical effects of anodic stimulation leading to facilitating the entry and exit of the motor system. These effects occur by increasing substances, such as myoinositol following tDCS and rTMS stimulation [50] inside the brain, which increases force production and its stability, or by decreasing mediators, such as gamma-aminobutyric acid (GABA), which compensates for impaired brain function [51]. Another mechanism for reducing fatigue during anodic stimulation is an increase in neuronal and axonal excitability in the cortex [52] which according to the pathophysiology of MS disease can be effective in axonal transmission. It can also be effective in facilitating thalamocortical afferents [53]. Resting-state MRI analysis showed that anodic stimulation strengthens neuronal connections, leading to recovery from fatigue [54, 55]. M1 stimulation increases cortical excitability and recruitment of more motor units and reduces supra-spinal fatigue [56]. In a review performed [57], the effectiveness of VR on functional mobility, balance, fatigue and quality of life in people with MS has been observed compared to regular exercises [58], which is consistent with our study. Previous studies have shown that the use of VR without accompanying physical exercises can be effective in improving the debilitating effects of MS disease [59]. Compared to physical exercises improving muscle resistance, heart rate and respiratory frequency plays a crucial role in the treatment of fatigue; VR is more adaptable to the patient’s conditions. The possibility of performing at home and high attractiveness in patients create more motivation.
On the other hand, the results of our study showed that in the post-test, the VR + tDCS group and the VR group had less fatigue than the tDCS group. Similarly, no improvement in fatigue symptoms was reported in active tDCS compared to sham+video game effect in a case report of MS patients.
The results showed that the VR+tDCS group as well as the VR group had better balance and showed a significant reduction in walking time from pre-test to post-test; while the tDCS groups did not show any significant change. Also, no significant difference was observed between the two VR+tDCS group and the VR group. The effectiveness of VR in the balance and gait of patients with MS compared to conventional mediated treatments has been proven due to their cost-effectiveness and attractiveness during review studies and meta-analysis [19, 60].
VR can provide a multiple sensory environment to the MS patient, which can cause neuroplasticity in the motor sensory cortex, and may be effective in the patient’s motor rehabilitation [61]. A similar effect of VR training and tDCS in promoting neuroplasticity of the cortex in neurologic patients has been shown by functional magnetic resonance imaging (fMRI) [62, 63].
Since several exergames, such as Xbox Kinect or Nintendo Wii sports games require fast hand-eye, and foot-eye coordination, VR training can improve cognitive-motor skills, including hand-eye coordination, and eye-foot coordination, and increase manual dexterity and the ability to perform movements like walking.
Therefore, we tried to use games in the present study in which coordination can play a significant role in playing them. For example, in the game of boxing, two-handed and hand-eye coordination, soccer game, eye-foot coordination, tennis game, eye-hand coordination, golf game, two-handed coordination, American football game, hand-eye coordination, and in skiing game, eye-foot coordination, eye-hand coordination, and bimanual coordination are essential.
To the best of our knowledge, except for a case study [39], this is the first randomized controlled study to apply a combined tDCS-VR method to MS patients. Although the facilitating effect of brain stimulation on the effectiveness of VR therapy has been reported in some previous studies [64, 65], we did not find a significant difference between the two groups of VR + tDCS combination with VR+sham, indicating that tDCS did not enhance the positive effect of VR on balance and walking speed. Consistent with our study, in some studies, the addition of tDCS did not strengthen the effect of VR [66]. The lack of difference between VR+ sham and VR+ anodal tDCS in the investigation conducted by Monte-Silva et al on stroke patients was explained by the ceiling effect [67]. The other reason may be that our tDCS protocol did not completely target the cortex area involved in balance and walking. Future studies are needed to evaluate the combined effect of placing anodal electrodes in other areas like Cz to stimulate the bilateral motor cortex.
Conclusion
Our results showed that VR separately and in combination with tDCS can improve fatigue, and balance and walking speed in patients with MS. However, we found more excessive effect in tDCS combined with VR therapy. Our results indicate that the effect of tDCS with VR therapy should be investigated further.
Considering that VR exercises and tDCS are a new training method of interventions for treating movement problems. And by creating an attractive and fresh environment, in addition to being a training environment, it makes people with MS cheerful and play a challenging environment by using games. It is suggested that according to the positive effects of VR and tDCS on significant or positive improvement in various parameters of this study, continuous use in the exercises of people with MS is suggested.
Also, due to the ease of using these exercises and the ability to do them at home and in rehabilitation centers, it is recommended to use these exercises at home and in rehabilitation centers.
It is recommended that evaluations be conducted for more accurate tests and instruments. For example, checking balance with Biodex and walking with quinoa, like examining plasticity by imaging the brain.
Considering that people with MS were used in this study, if possible, it should be tested for other people who have movement problems.
In this research, due to the limitations of the subject and the researcher, limited variables were measured. It is recommended to examine other movement variables related to MS, such as coordination and reaction time.
Ethical Considerations
Compliance with ethical guidelines
The present study was conducted according to the Helsinki Ethics Statement, and also the Ethics Committee of the School of Physical Education and Sport Science at the University of Tehran approved the study protocol (Code: IR.UT.SPORT.REC.1400.034). Participants entered the study after completing the written informed consent form and had the option to withdraw from the research at any time.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the patients with MS who participated in the study.
References
Multiple sclerosis (MS) is a chronic and degenerative neurological disease, with myelin sheath damage in the central nervous system (CNS) [1]. Pathological features of the disease, such as inflammation, demyelination, and destruction of neurons differently appear [2]. The most common non-traumatic CNS injury in young adults with an average age of 20-40 years is MS [3]. The clinical course of the disease is heterogeneous, including mild neurological symptoms to progressive and debilitating forms [3]. Due to the demyelination, patients experience simple or multiple sensory, motor, emotional-behavioral, and cognitive symptoms [4], depending on the location of the lesions [5]. Common symptoms of the disease include muscle weakness, spasticity, fatigue, sensory dysfunctions, balance problems, cognitive disorders, and difficulty in walking [6] Symptoms significantly affect a patient’s social communication, quality of life, job, and performance [7].
Fatigue, as one of the common and debilitating symptoms [8], affects about 80% of patients at different stages of the disease [9]. It reduces cognitive and functional abilities, associated with increased need for rest and decreased motivation [10]. Primary and secondary progressive stages of the disease showed more fatigue symptoms compared to relapsing-remitting MS [4]. The pathophysiology of fatigue in MS is not known [11], but it occurs as a result of brain dysfunction (primary fatigue) or following symptoms during the disease (secondary fatigue) [12]. Primary fatigue results from the pathophysiological process of the disease itself, and also secondary fatigue is caused by the complications of the disease, such as endocrine disorders, infections, lack of vitamins, and anemia [8, 10]. People with MS can have limitations in daily activities due to muscle weakness, spasticity, and imbalance. Therefore, it has a bad effect on walking and increases the risk of falling. More than spasticity in MS people, it prevents functional activities, such as moving, which increases disability. Therefore, fatigue has a negative effective (mentally and physically) life and the ability to work in patients with MS by limiting daily activities and coping abilities [13, 14].
Imbalance is one of the primary symptoms of MS associated with an increased risk of falling [15, 16]. Reduced functional capacity, muscle weakness, fatigue, and spasms, which are common symptoms in these patients, may lead to inappropriate balance [17, 18]. This debilitating symptom has been reported in 75% of patients, which can reduce the mobility and independence of the affected person and ultimately affect his/her quality of life [19]. Balance problems occur at the beginning of the disease and usually increase with the progress of the disease [20].
Walking disorder is also commonly seen in MS patients and is described as the most challenging symptom by 70% of patients [21, 22]. The patterns of walking disorders that are reflected as asymmetry and coordination dysfunction in patients reduce walking speed and increase energy consumption [23]. Walking disorders appear in different ways, including decreasing the length of the step, reducing the speed, and increasing the width of the step [24]. Walking and balance problems lead to an increase in falling risk, activity limitation, and isolation, which are worsened by the progression of the disease [25].
Pharmacological treatment controls the symptoms and consequences of the disease, but the results are not clinically satisfactory. Alternative therapies have introduced to relieve existing symptoms and to prevent complications of MS [26].
Exercise training and behavioral therapy are both useful for patients with mild disease symptoms [1]. In recent years, in addition to physical exercises, the number of studies on the effectiveness of other non-drug methods, including non-invasive brain stimulation techniques, has increased. One of these effective methods is transcranial direct current electrical stimulation (tDCS), which is easy to use, and is considered a cheap and non-invasive tool for the motor rehabilitation of patients [6, 25]. tDCS produces a low-amplitude direct current that can change cortical excitability without harmful side effects [2, 8]. Stimulation with anodic direct current increases the resting potential of the neuronal membrane, while with cathode current resting potential [26].
tDCS is widely used in the rehabilitation of various neurological diseases, such as stroke, Parkinson’s disease, and MS [27]. Navarra-Lopez et al. in their systematic review reported that tDCS along with physical therapy can improve the walking parameters, static and dynamic balance, and lower limb function in stroke [28]. Another study conducted on people with Parkinson’s disease in 2020 showed that one session of bilateral and electrical stimulation of the cerebellum can significantly improve balance performance [29].
Among other techniques in rehabilitation, virtual reality (VR), focusing on neural augmentation and motor learning is an effective method that can be used as an alternative to traditional rehabilitation in MS patients [1]. VR provides the user with an alternative and favorable simulation of activity or environment that permits interaction through multiple sensory systems [30]. The environment contains various stimulation which produces a potent signal to reorganize sensorimotor circuits which can affect the motor cortex during motor learning [31]. By the way, repeated and purposeful observation of activities can influence cortico-cortical interactions in the premotor and motor areas [32]. Therefore VR prepares relevant and meaningful stimulation to individual’s different brain areas, promoting motor learning and rehabilitation via neuroplasticity. VR not only improves patients’ quality of life but also has a role in the return of their brain health [33]. In recent years, VR technology has increasingly become affordable, flexible, and portable, which enables researchers to consider its use in many fields, especially the medical field [34, 35]. In a systematic study and meta-analysis, Zhang et al. confirmed that VR training is effective in the motor function of the upper and lower limbs, walking, balance, and daily activity of stroke patients and improves variables, but without effect on cognition [36]. Moreover, the study conducted by Abou L et al showed positive effects of VR training that improves balance during sitting and standing and a trend of gait improvement in persons with SCI [37]. Another study performed by Wang et al. [38] on the effectiveness of VR on the balance and walking of people with Parkinson’s showed a significant effect on balance with no effect on walking [38].
The limited studies evaluated the effects of tDCS and VR on complications, such as fatigue, balance disorder, and walking in people with MS. Therefore, the present study was conducted to evaluate the effect of tDCS and VR on the mentioned symptoms of the disease. In addition, no investigation was found to study the combined effects of VR and tDCS on the mentioned variables. The synergistic effects of these two intervention methods can lead to greater effectiveness and shorter treatment time. Therefore, the secondary goal of this investigation was to compare the therapeutic effects of VR and tDCS separately and in combination on the level of fatigue, balance, and some walking parameters (speed & stride length) of people with MS.
Materials and Methods
The participants included 30 patients with MS aged 18-55 years. Patients whose disease was confirmed by neurologists participated in the present study voluntarily and purposefully. The inclusion criteria included a patient with expanded disability status scale (EDSS) ≤6 (EDSS is used to quantify disability and monitor its alterations during the time in MS) and not having any type of severe visual impairment, no history of concussion, ability to walk independently with or without an assistive device and not having any attack during last month. By the way, the exclusion criteria included patients with any severe systemic disorder, such as epilepsy or psychiatric disease, which prevented the use of VR modality and electrical stimulation. Participants entered the study after completing the written informed consent form and had the option to withdraw from the research at any time. The patients were brain type of MS with their routine MS drug therapy without participating in other rehabilitation interventions. Table 1 presents the demographic information of the subjects.

The present study is a clinical trial study, with pre and post-assessment. The subjects were randomly allocated (by choosing a number from the box) to one of three groups according to the study inclusion/exclusion criteria, tDCS-VR group (n=10), VR group (n=10), and tDCS group (n=10).
The pre-test performed before the intervention included the fatigue severity scale (FSS), modified fatigue effect scale (MFIS), balance sheet scale (BBS), and 25-foot walk test (T25-FW). Immediately after the intervention, these evaluations were repeated as post-test. The intervention stages are shown in Figure 1. The researcher administering all tests was blinded to the test condition.

tDCS
A device (ActivaDose) was used to administer tDCS by a trained therapist. At first, we placed the anode and cathode electrodes in 5×5 cm sponge pads, and the pads were dipped in salt solution. We placed the anode electrode on the motor cortex M1 (C3) on the left side and the cathode on the right forehead according to the international 10-20 system [38]. Then, the stimulation protocol [38] consisting of electrical stimulation of the brain with a direct current of 2 mA, 20 minutes daily for 5 consecutive days was used. As a sham intervention, the current was turned off after 60 s of the onset of stimulation.
VR
The VR protocol was implemented using the VR BOX headset and based on the defined protocol [17, 39] and Costa (2020) [40]. Patients performed the VR program three sessions per week for two weeks. The duration of each session was 20 minutes.
We used the VR BOX headset to implement the VR protocol. In this way, we first put the Android phone inside the headset compartment and then put it on the patient’s face.
The combined protocol was as follows: We placed the anode and cathode electrodes on the M1 motor cortex and on the forehead, respectively. We applied direct electrical stimulation of the brain for two weeks and three seasons every week with a current intensive of 2MA and 20 minutes. After fixing the electrodes in the desired place using a strap, we placed the VR headset on the patient’s face and applied two stimuli simultaneously. VR was also performed for six sessions, three times a week, and for 20 minutes.
The games include Slalom ski, penguin slide, and marble balance:
Slalom ski: This game requires the patient to pass between two flags placed along the track.
Penguin slide: In this game, patients must move from the ice platform to the fishing points outside the platform without falling.
Marble balance: In this game, the patient has to put the ball in a hole. Accordingly, he/she should shift his/her body weight to the direction where he/she wants the ball to be placed. If successful, the patient is transferred to the next stage, which is more difficult.
It should be noted that the VR group was also considered as a sham group and thus therefore simultaneously received sham stimulation by electrodes located according to the mentioned protocol.
During the combined VR+tDCS protocol, the anode and cathode electrodes were placed on the M1 motor cortex and the forehead, respectively, and at the same time, the VR headset was placed on a patient’s face, and both types of intervention were performed simultaneously (tDCS with intensity of 2 mA that simultaneously applied using VR for 20 minutes for two weeks and three sessions each per week).
Tools
FSS: FSS is a 9-item scale that mainly focuses on the physical aspect of fatigue. Each item has a 7-point Likert scale, ranging from 1 (completely disagree) to 7 (completely agree). Therefore, the total score can be from 9 to 63. Having a FSS <4, 4> FSS <5, and FSS >5 is classified as mild, moderate, and severe fatigue, respectively. In the Iranian version of the instrument, Cronbach’s α coefficient and intra-class correlation coefficient (ICC) were reported as 0.96, and 0.93 respectively. A validity of 85% was shown in the researchers’ results [40].
MFIS: MFIS is a 21-item scale that assesses cognitive and psychosocial in addition to physical dimensions with a 5-point Likert scale, ranging from zero (never) to four (almost always). The range of total score is from 0 to 84. A correlation of r=68%, P<0.0001 was reported between the two tests of fatigue intensity scale and modified fatigue effect [41]. A high Cronbach’s score and ICC were reported in the Persian version of MFIS [41].
Berg balance scale (BBS): BBS contains 14 common functional activities that are performed repeatedly in a day in a 4-point Likert scale, ranging from 0 to 4 (0 indicating the lowest level of performance and 4 indicating the highest level of performance). Persian version of the test showed very high inter-rater and test re-test reliability (ICC=0.93 and 0.95, respectively) in the elderly [42, 43].
T 25-F W: T 25-F W as one of the three components of patients with MS function evaluates walking speed. Walking speed in meters per second is obtained by dividing the measured distance of 25 feet by the time. The reliability and validity of the T25-FW is reported as 97% [44].
Statistical analysis
The first normality of data was checked. The dependent t-test was used to compare within-group variables. Then analysis of covariance (ANCOVA) was employed to determine differences between the post-test scores of three groups, using baseline values as covariates while Turkey’s method was used as a post-test. P<0.05 was regarded as statistically significant. SPSS software, version 19, was used for statistical analysis.
Results
All participants completed all stages, and also no drop was observed in the number of samples in the present study. Table 1 presents the demographic characteristics and disability score (Mean±SD) of the groups. Descriptive finding and comparison of within-group assessments were shown in Table 2.

The one-way analysis of covariance (ANCOVA) was used to compare the adjusted mean of the dependent variables in the 2×3 design (three groups in two stages). A significant difference was observed between the post-test-adjusted mean of all dependent variables (P<0.05) (Table 3).

According to Turkey’s post hoc test results of the mean intensity of fatigue, both the VR and the tDCS-VR group significantly had lower scores than the tDCS group (P<0.001); however, the mean fatigue intensity between the VR and the tDCS-VR group was not significant (P=0.990) (Table 4).
Regarding the post hoc results of the balance test, the VR and tDCS-VR groups were significantly better than the tDCS group (P<0.001). No significant difference was observed between the mean balance of the VR group and the tDCS-VR group (P=0.995) (Table 4).

According to the mean walking speed, the VR and the tDCS-VR groups were significantly better than the tDCS group (P<0.001). While the difference between the mean walking speed of the VR and the tDCS-VR groups was not significant (P=1.000).
Moreover, in the mean modified fatigue, the VR group significantly showed lower scores than the tDCS and tDCS-VR groups (P=0.037), therefore it can be said that the VR intervention significantly reduced the modified fatigue compared to the other two interventions.
Discussion
The present study was conducted to investigate the effects of VR and tDCS separately and in combination on fatigue; balance and walking speed of MS patients. The results showed a significant effect of tDCS, VR, and tDCS-VR on fatigue and a significant effect of VR and tDCS-VR on balance and walking speed in patients with MS. In comparing the effect of different interventions on fatigue, balance, and walking speed, the VR and tDCS-VR groups significantly showed better results, but no significant difference was observed between VR and tDCS-VR groups. A significant difference of the modified fatigue was observed between the VR with the other groups.
Fatigue: Our study showed that all three groups (including AtDCS, VR, and tDCS-VR) had a significant decrease in the intensity of fatigue in the post-test compared to the pre-test. The participants in the tDCS group improved severe fatigue symptoms and 8 of them improved the modified fatigue. In the present study, anodic stimulation was used by placing the stimulating electrode on the left M1 region and the cathode on the right frontal region. In previous studies, different areas have been used to reduce fatigue, such as [45] dorsolateral prefrontal cortex (DLPFC), parietal P4 [46] and S1 [47] and M1 [48]. Anodic stimulation can improve fatigue caused by MS by several mechanisms, including the antidepressant effects of AtDCS [49], and the neurochemical effects of anodic stimulation leading to facilitating the entry and exit of the motor system. These effects occur by increasing substances, such as myoinositol following tDCS and rTMS stimulation [50] inside the brain, which increases force production and its stability, or by decreasing mediators, such as gamma-aminobutyric acid (GABA), which compensates for impaired brain function [51]. Another mechanism for reducing fatigue during anodic stimulation is an increase in neuronal and axonal excitability in the cortex [52] which according to the pathophysiology of MS disease can be effective in axonal transmission. It can also be effective in facilitating thalamocortical afferents [53]. Resting-state MRI analysis showed that anodic stimulation strengthens neuronal connections, leading to recovery from fatigue [54, 55]. M1 stimulation increases cortical excitability and recruitment of more motor units and reduces supra-spinal fatigue [56]. In a review performed [57], the effectiveness of VR on functional mobility, balance, fatigue and quality of life in people with MS has been observed compared to regular exercises [58], which is consistent with our study. Previous studies have shown that the use of VR without accompanying physical exercises can be effective in improving the debilitating effects of MS disease [59]. Compared to physical exercises improving muscle resistance, heart rate and respiratory frequency plays a crucial role in the treatment of fatigue; VR is more adaptable to the patient’s conditions. The possibility of performing at home and high attractiveness in patients create more motivation.
On the other hand, the results of our study showed that in the post-test, the VR + tDCS group and the VR group had less fatigue than the tDCS group. Similarly, no improvement in fatigue symptoms was reported in active tDCS compared to sham+video game effect in a case report of MS patients.
The results showed that the VR+tDCS group as well as the VR group had better balance and showed a significant reduction in walking time from pre-test to post-test; while the tDCS groups did not show any significant change. Also, no significant difference was observed between the two VR+tDCS group and the VR group. The effectiveness of VR in the balance and gait of patients with MS compared to conventional mediated treatments has been proven due to their cost-effectiveness and attractiveness during review studies and meta-analysis [19, 60].
VR can provide a multiple sensory environment to the MS patient, which can cause neuroplasticity in the motor sensory cortex, and may be effective in the patient’s motor rehabilitation [61]. A similar effect of VR training and tDCS in promoting neuroplasticity of the cortex in neurologic patients has been shown by functional magnetic resonance imaging (fMRI) [62, 63].
Since several exergames, such as Xbox Kinect or Nintendo Wii sports games require fast hand-eye, and foot-eye coordination, VR training can improve cognitive-motor skills, including hand-eye coordination, and eye-foot coordination, and increase manual dexterity and the ability to perform movements like walking.
Therefore, we tried to use games in the present study in which coordination can play a significant role in playing them. For example, in the game of boxing, two-handed and hand-eye coordination, soccer game, eye-foot coordination, tennis game, eye-hand coordination, golf game, two-handed coordination, American football game, hand-eye coordination, and in skiing game, eye-foot coordination, eye-hand coordination, and bimanual coordination are essential.
To the best of our knowledge, except for a case study [39], this is the first randomized controlled study to apply a combined tDCS-VR method to MS patients. Although the facilitating effect of brain stimulation on the effectiveness of VR therapy has been reported in some previous studies [64, 65], we did not find a significant difference between the two groups of VR + tDCS combination with VR+sham, indicating that tDCS did not enhance the positive effect of VR on balance and walking speed. Consistent with our study, in some studies, the addition of tDCS did not strengthen the effect of VR [66]. The lack of difference between VR+ sham and VR+ anodal tDCS in the investigation conducted by Monte-Silva et al on stroke patients was explained by the ceiling effect [67]. The other reason may be that our tDCS protocol did not completely target the cortex area involved in balance and walking. Future studies are needed to evaluate the combined effect of placing anodal electrodes in other areas like Cz to stimulate the bilateral motor cortex.
Conclusion
Our results showed that VR separately and in combination with tDCS can improve fatigue, and balance and walking speed in patients with MS. However, we found more excessive effect in tDCS combined with VR therapy. Our results indicate that the effect of tDCS with VR therapy should be investigated further.
Considering that VR exercises and tDCS are a new training method of interventions for treating movement problems. And by creating an attractive and fresh environment, in addition to being a training environment, it makes people with MS cheerful and play a challenging environment by using games. It is suggested that according to the positive effects of VR and tDCS on significant or positive improvement in various parameters of this study, continuous use in the exercises of people with MS is suggested.
Also, due to the ease of using these exercises and the ability to do them at home and in rehabilitation centers, it is recommended to use these exercises at home and in rehabilitation centers.
It is recommended that evaluations be conducted for more accurate tests and instruments. For example, checking balance with Biodex and walking with quinoa, like examining plasticity by imaging the brain.
Considering that people with MS were used in this study, if possible, it should be tested for other people who have movement problems.
In this research, due to the limitations of the subject and the researcher, limited variables were measured. It is recommended to examine other movement variables related to MS, such as coordination and reaction time.
Ethical Considerations
Compliance with ethical guidelines
The present study was conducted according to the Helsinki Ethics Statement, and also the Ethics Committee of the School of Physical Education and Sport Science at the University of Tehran approved the study protocol (Code: IR.UT.SPORT.REC.1400.034). Participants entered the study after completing the written informed consent form and had the option to withdraw from the research at any time.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the patients with MS who participated in the study.
References
- Ashrafi A, Mohseni-Bandpei MA, Seydi M. The effect of tDCS on the fatigue in patients with multiple sclerosis: A systematic review of randomized controlled clinical trials. Journal of Clinical Neuroscience. 2020; 78:277-83. [DOI:10.1016/j.jocn.2020.04.106] [PMID]
- Charvet LE, Dobbs B, Shaw MT, Bikson M, Datta A, Krupp LB. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: Results from a randomized, sham-controlled trial. Multiple Sclerosis. 2018; 24(13):1760-9. [DOI:10.1177/1352458517732842] [PMID]
- Farid R, Norasteh A, Hatamian H. The effect of core stability exercise program on the balance of patients with multiple sclerosis. Caspian Journal of Neurological Sciences. 2016; 2(1):9-17. [DOI:10.18869/acadpub.cjns.2.4.9]
- Ayache SS, Chalah MA. Transcranial direct current stimulation: A glimmer of hope for multiple sclerosis fatigue? Journal of Clinical Neuroscience. 2018; 55:10-12. [DOI:10.1016/j.jocn.2018.06.002] [PMID]
- Dobson R, Giovannoni G. Multiple sclerosis - A review. European Journal of Neurology. 2019; 26(1):27-40. [DOI:10.1111/ene.13819] [PMID]
- Pilloni G, Choi C, Coghe G, Cocco E, Krupp LB, Pau M, et al. Gait and functional mobility in multiple sclerosis: Immediate effects of transcranial direct current stimulation (tDCS) paired with aerobic exercise. Frontiers in Neurology. 2020; 11:310. [DOI:10.3389/fneur.2020.00310] [PMID]
- Hsu WY, Cheng CH, Zanto TP, Gazzaley A, Bove RM. Effects of transcranial direct current stimulation on cognition, mood, pain, and fatigue in multiple sclerosis: A systematic review and meta-analysis. Frontiers in Neurology. 2021; 12:626113. [DOI:10.3389/fneur.2021.626113] [PMID]
- Adibi I, Sanayei M, Tabibian F, Ramezani N, Pourmohammadi A, Azimzadeh K. Multiple sclerosis-related fatigue lacks a unified definition: A narrative review. Journal of Research in Medical Sciences. 2022; 27:24. [DOI:10.4103/jrms.jrms_1401_20] [PMID]
- Johansson S, Skjerbæk AG, Nørgaard M, Boesen F, Hvid LG, Dalgas U. Associations between fatigue impact and lifestyle factors in people with multiple sclerosis - The Danish MS hospitals rehabilitation study. Multiple Sclerosis and Related Disorders. 2021; 50:102799. [DOI:10.1016/j.msard.2021.102799] [PMID]
- Moore H, Nair KPS, Baster K, Middleton R, Paling D, Sharrack B. Fatigue in multiple sclerosis: A UK MS-register based study. Multiple Sclerosis and Related Disorders. 2022; 64:103954. [DOI:10.1016/j.msard.2022.103954] [PMID]
- Massetti T, Trevizan IL, Arab C, Favero FM, Ribeiro-Papa DC, de Mello Monteiro CB. Virtual reality in multiple sclerosis - A systematic review. Multiple Sclerosis and Related Disorders. 2016; 8:107-12. [DOI:10.1016/j.msard.2016.05.014] [PMID]
- Veldhuijzen van Zanten J, Douglas MR, Ntoumanis N. Fatigue and fluctuations in physical and psychological wellbeing in people with multiple sclerosis: A longitudinal study. Multiple Sclerosis and Related Disorders. 2021; 47:102602. [DOI:10.1016/j.msard.2020.102602] [PMID]
- Fiene M, Rufener KS, Kuehne M, Matzke M, Heinze HJ, Zaehle T. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. Journal of Neurology. 2018; 265(3):607-17. [DOI:10.1007/s00415-018-8754-6] [PMID]
- Heitmann H, Andlauer TFM, Korn T, Mühlau M, Henningsen P, Hemmer B, et al. Fatigue, depression, and pain in multiple sclerosis: How neuroinflammation translates into dysfunctional reward processing and anhedonic symptoms. Multiple Sclerosis. 2022; 28(7):1020-7. [DOI:10.1177/1352458520972279] [PMID]
- Ozkul C, Guclu-Gunduz A, Yazici G, Guzel NA, Irkec C. Effect of immersive virtual reality on balance, mobility, and fatigue in patients with multiple sclerosis: A single-blinded randomized controlled trial. European Journal of Integrative Medicine. 2020; 35:101092. [DOI:10.1016/j.eujim.2020.101092]
- Cusin FS, Tomaz A, Ganança MM, Oliveira EM, Gonçalves ABF, Caovilla HH. Postural control in relapsing-remitting multiple sclerosis. International Archives of Otorhinolaryngology. 2022; 26(4):e592-604. [DOI:10.1055/s-0041-1741026] [PMID]
- Yazgan YZ, Tarakci E, Tarakci D, Ozdincler AR, Kurtuncu M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: A randomized controlled trial. Multiple Sclerosis and Related Disorders. 2020; 39:101902. [DOI:10.1016/j.msard.2019.101902] [PMID]
- Arntzen EC, Straume BK, Odeh F, Feys P, Zanaboni P, Normann B. Group-based individualized comprehensive core stability intervention improves balance in persons with multiple sclerosis: A randomized controlled trial. Physical Therapy. 2019; 99(8):1027-38. [DOI:10.1093/ptj/pzz017] [PMID]
- Casuso-Holgado MJ, Martín-Valero R, Carazo AF, Medrano-Sánchez EM, Cortés-Vega MD, Montero-Bancalero FJ. Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: A systematic review and meta-analysis. Clinical Rehabilitation. 2018; 32(9):1220-34. [DOI:10.1177/0269215518768084] [PMID]
- Alghwiri AA, Khalil H, Al-Sharman A, El-Salem K. Depression is a predictor for balance in people with multiple sclerosis. Multiple Sclerosis and Related Disorders. 2018; 24:28-31. [DOI:10.1016/j.msard.2018.05.013] [PMID]
- Pilloni G, Choi C, Shaw MT, Coghe G, Krupp L, Moffat M, et al. Walking in multiple sclerosis improves with tDCS: A randomized, double-blind, sham-controlled study. Annals of Clinical and Translational Neurology. 2020; 7(11):2310-19. [DOI:10.1002/acn3.51224] [PMID]
- Sikes EM, Cederberg KL, Sandroff BM, Bartolucci A, Motl RW. Quantitative synthesis of timed 25-foot walk performance in multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2020; 101(3):524-34. [DOI:10.1016/j.apmr.2019.08.488] [PMID]
- Plotnik M, Wagner JM, Adusumilli G, Gottlieb A, Naismith RT. Gait asymmetry, and bilateral coordination of gait during a six-minute walk test in persons with multiple sclerosis. Scientific Reports. 2020; 10(1):12382. [DOI:10.1038/s41598-020-68263-0] [PMID]
- Coca-Tapia M, Cuesta-Gómez A, Molina-Rueda F, Carratalá-Tejada M. Gait pattern in people with multiple sclerosis: A systematic review. Diagnostics. 2021; 11(4):584. [DOI:10.3390/diagnostics11040584] [PMID]
- Chalah MA, Grigorescu C, Padberg F, Kümpfel T, Palm U, Ayache SS. Bifrontal transcranial direct current stimulation modulates fatigue in multiple sclerosis: A randomized sham-controlled study. Journal of Neural Transmission. 2020; 127(6):953-61. [DOI:10.1007/s00702-020-02166-2] [PMID]
- Borisow N, Döring A, Pfueller CF, Paul F, Dörr J, Hellwig K. Expert recommendations to personalization of medical approaches in treatment of multiple sclerosis: An overview of family planning and pregnancy. The EPMA Journal. 2012; 3(1):9. [DOI:10.1186/1878-5085-3-9] [PMID]
- Young J, Zoghi M, Khan F, Galea MP. The effect of transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis: Randomized controlled trial. Pain Medicine. 2020; 21(12):3451-7. [DOI:10.1093/pm/pnaa128] [PMID]
- Navarro-López V, Molina-Rueda F, Jiménez-Jiménez S, Alguacil-Diego IM, Carratalá-Tejada M. Effects of transcranial direct current stimulation combined with physiotherapy on gait pattern, balance, and functionality in stroke patients. A systematic review. Diagnostics. 2021; 11(4):656. [DOI:10.3390/diagnostics11040656] [PMID]
- Workman CD, Kamholz J, Rudroff T. Transcranial direct current stimulation (tDCS) to improve gait in multiple sclerosis: A timing window comparison. Frontiers in Human Neuroscience. 2019; 13:420. [DOI:10.3389/fnhum.2019.00420] [PMID]
- Workman CD, Fietsam AC, Uc EY, Rudroff T. Cerebellartranscranial direct current stimulation in people with parkinson’s disease: A pilot study. Brain Sciences. 2020; 10(2):96. [DOI:10.3390/brainsci10020096] [PMID]
- Adamovich SV, Fluet GG, Tunik E, Merians AS. Sensorimotor training in virtual reality: A review. NeuroRehabilitation. 2009; 25(1):29-44. [DOI:10.3233/NRE-2009-0497] [PMID]
- Bray S, Shimojo S, O’Doherty JP. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. The Journal of Neuroscience. 2007; 27(28):7498-507. [DOI:10.1523/JNEUROSCI.2118-07.2007] [PMID]
- Léonard G, Tremblay F. Corticomotor facilitation associated with observation, imagery and imitation of hand actions: A comparative study in young and old adults. Experimental Brain Research. 2007; 177(2):167-75. [DOI:10.1007/s00221-006-0657-6] [PMID]
- ramaki AL, Sampaio RF, Reis ACS, Cavalcanti A, Dutra FCMSE. Virtual reality in the rehabilitation of patients with stroke: An integrative review. Arquivos de Neuro-Psiquiatria. 2019; 77(4):268-78. [DOI:10.1590/0004-282x20190025] [PMID]
- Ioannou A, Papastavrou E, Avraamides MN, Charalambous A. Virtual reality and symptoms management of anxiety, depression, fatigue, and pain: A systematic review. SAGE Open Nursing. 2020; 6:2377960820936163. [DOI:10.1177/2377960820936163] [PMID]
- Zhang B, Li D, Liu Y, Wang J, Xiao Q. Virtual reality for limb motor function, balance, gait, cognition and daily function of stroke patients: A systematic review and meta-analysis. Journal of Advanced Nursing. 2021; 77(8):3255-73. [DOI:10.1111/jan.14800] [PMID]
- Abou L, Malala VD, Yarnot R, Alluri A, Rice LA. Effects of virtual reality therapy on gait and balance among individuals with spinal cord injury: A systematic review and meta-analysis. Neurorehabilitation and Neural Repair. 2020; 34(5):375-88. [DOI:10.1177/1545968320913515] [PMID]
- Wang B, Shen M, Wang YX, He ZW, Chi SQ, Yang ZH. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clinical Rehabilitation. 2019; 33(7):1130-8. [DOI:10.1177/0269215519843174] [PMID]
- Mortezanejad M, Ehsani F, Masoudian N, Zoghi M, Jaberzadeh S. Comparing the effects of multi-session anodal trans-cranial direct current stimulation of primary motor and dorsolateral prefrontal cortices on fatigue and quality of life in patients with multiple sclerosis: A double-blind, randomized, sham-controlled trial. Clinical Rehabilitation. 2020; 34(8):1103-11. [DOI:10.1177/0269215520921506] [PMID]
- Costa GC, Kunitake AI, Fonseca Junior PR, Ledur ÂC, Elord Júlio C, Pereira GS, et al. Effect of transcranial direct current stimulation combined with a virtual reality exercise on balance in a patient with multiple sclerosis: A case report. Adaptive Behavior. 2020; 28(4):307-13. [DOI:10.1177/1059712319873912]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989; 46(10):1121-3. [DOI:10.1001/archneur.1989.00520460115022] [PMID]
- Ghajarzadeh M, Jalilian R, Eskandari G, Ali Sahraian M, Reza Azimi A. Validity and reliability of Persian version of modified fatigue impact scale (MFIS) questionnaire in Iranian patients with multiple sclerosis. Disability and Rehabilitation. 2013; 35(18):1509-12. [DOI:10.3109/09638288.2012.742575] [PMID]
- Salavati M, Negahban H, Mazaheri M, Soleimanifar M, Hadadi M, Sefiddashti L, et al. The Persian version of the berg balance scale: Inter and intra-rater reliability and construct validity in elderly adults. Disability and Rehabilitation. 2012; 34(20):1695-8. [DOI:10.3109/09638288.2012.660604] [PMID]
- Motl RW, Cohen JA, Benedict R, Phillips G, LaRocca N, Hudson LD, et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Multiple Sclerosis. 2017; 23(5):704-10. [DOI:10.1177/1352458517690823] [PMID]
- Chalah MA, Riachi N, Ahdab R, Mhalla A, Abdellaoui M, Créange A, et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. Journal of the Neurological Sciences. 2017; 372:131-7. [DOI:10.1016/j.jns.2016.11.015] [PMID]
- Hanken K, Bosse M, Möhrke K, Eling P, Kastrup A, Antal A, et al. Counteracting fatigue in multiple sclerosis with right parietal anodal transcranial direct current stimulation. Frontiers in Neurology. 2016; 7:154. [DOI:10.3389/fneur.2016.00154]
- Tecchio F, Cancelli A, Cottone C, Ferrucci R, Vergari M, Zito G, et al. Brain plasticity effects of neuromodulation against multiple sclerosis fatigue. Frontiers in Neurology. 2015; 6:141. [DOI:10.3389/fneur.2015.00141] [PMID]
- Ferrucci R, Vergari M, Cogiamanian F, Bocci T, Ciocca M, Tomasini E, et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. 2014; 34(1):121-7. [DOI:10.3233/NRE-131019] [PMID]
- Brunoni AR, Ferrucci R, Fregni F, Boggio PS, Priori A. Transcranial direct current stimulation for the treatment of major depressive disorder: A summary of preclinical, clinical and translational findings. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012; 39(1):9-16. [DOI:10.1016/j.pnpbp.2012.05.016] [PMID]
- Rango M, Cogiamanian F, Marceglia S, Barberis B, Arighi A, Biondetti P, et al. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: A 1H-MRS study. Magnetic Resonance in Medicine. 2008; 60(4):782-9. [DOI:10.1002/mrm.21709] [PMID]
- Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Experimental Brain Research. 2007; 179(2):255-62. [DOI:10.1007/s00221-006-0790-2] [PMID]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Supplements to Clinical Neurophysiology. 2003; 56:255-76. [DOI:10.1016/S1567-424X(09)70230-2] [PMID]
- Leocani L, Colombo B, Magnani G, Martinelli-Boneschi F, Cursi M, Rossi P, et al. Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement--EEG evidence. Neuroimage. 2001; 13(6 Pt 1):1186-92. [DOI:10.1006/nimg.2001.0759] [PMID]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. The Journal of Neuroscience. 2011; 31(43):15284-93. [DOI:10.1523/JNEUROSCI.0542-11.2011] [PMID]
- Polanía R, Paulus W, Nitsche MA. Reorganizing the intrinsic functional architecture of the human primary motor cortex during rest with non-invasive cortical stimulation. Plos One. 2012; 7(1):e30971. [DOI:10.1371/journal.pone.0030971] [PMID]
- Alix-Fages C, Romero-Arenas S, Castro-Alonso M, Colomer-Poveda D, Río-Rodriguez D, Jerez-Martínez A, et al. Short-term effects of anodal transcranial direct current stimulation on endurance and maximal force production. A systematic review and meta-analysis. Journal of Clinical Medicine. 2019; 8(4):536. [DOI:10.3390/jcm8040536] [PMID]
- Murru V, Farris E, Santo A, Grillo O, Piazza C, Gaio A, et al. Niche differentiation at multiple spatial scales on large and small mediterranean islands for the endemic silene velutina pourr. ex Loisel. (Caryophyllaceae). Plants. 2021; 10(11):2298. [DOI:10.3390/plants10112298] [PMID]
- Cortés-Pérez I, Sánchez-Alcalá M, Nieto-Escámez FA, Castellote-Caballero Y, Obrero-Gaitán E, Osuna-Pérez MC. Virtual reality-based therapy improves fatigue, impact, and quality of life in patients with multiple sclerosis. A systematic review with a meta-analysis. Sensors. 2021; 21(21):7389. [DOI:10.3390/s21217389] [PMID]
- Castellano-Aguilera A, Biviá-Roig G, Cuenca-Martínez F, Suso-Martí L, Calatayud J, Blanco-Díaz M, et al. Effectiveness of virtual reality on balance and risk of falls in people with multiple sclerosis: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):14192. [DOI:10.3390/ijerph192114192] [PMID]
- Cheung KL, Tunik E, Adamovich SV, Boyd LA. Neuroplasticity and virtual reality. In: Weiss PLT, Keshner EA, Levin MF, editors. Virtual reality for physical and motor rehabilitation. Berlin: Springer; 2014. [DOI:10.1007/978-1-4939-0968-1_2]
- Kim YJ, Ku J, Cho S, Kim HJ, Cho YK, Lim T, et al. Facilitation of corticospinal excitability by virtual reality exercise following anodal transcranial direct current stimulation in healthy volunteers and subacute stroke subjects. Journal of Neuroengineering and Rehabilitation. 2014; 11:124. [DOI:10.1186/1743-0003-11-124] [PMID]
- You SH, Jang SH, Kim YH, Hallett M, Ahn SH, Kwon YH, et al. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke. 2005; 36(6):1166-71. [DOI:10.1161/01.STR.0000162715.43417.91] [PMID]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? The European Journal of Neuroscience. 2005; 22(2):495-504. [DOI:10.1111/j.1460-9568.2005.04233.x] [PMID]
- Teo WP, Muthalib M, Yamin S, Hendy AM, Bramstedt K, Kotsopoulos E, et al. Does a combination of virtual reality, neuromodulation and neuroimaging provide a comprehensive platform for neurorehabilitation? A narrative review of the literature. Frontiers in Human Neuroscience. 2016; 10:284. [DOI:10.3389/fnhum.2016.00284] [PMID]
- Harris DM, Rantalainen T, Muthalib M, Johnson L, Duckham RL, Smith ST, et al. Concurrent exergaming and transcranial direct current stimulation to improve balance in people with Parkinson’s disease: Study protocol for a randomised controlled trial. Trials. 2018; 19(1):387. [DOI:10.1186/s13063-018-2773-6] [PMID]
- Langbehn E, Steinicke F, Koo-Poeggel P, Marshall L, Bruder G. Stimulating the brain in VR: Effects of transcranial direct-current stimulation on redirected walking. ACM Symposium on Applied Perception. 2019; 10:1-9. [DOI:10.1145/3343036.3343125]
- Viana RT, Laurentino GE, Souza RJ, Fonseca JB, Silva Filho EM, Dias SN, et al. Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: A pilot randomized controlled trial. NeuroRehabilitation. 2014; 34(3):437-46. [DOI:10.3233/NRE-141065] [PMID]
Type of Study: Research |
Subject:
General
Received: 2023/08/7 | Accepted: 2024/01/3 | Published: 2024/07/1
Received: 2023/08/7 | Accepted: 2024/01/3 | Published: 2024/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






