Sat, Dec 20, 2025
Volume 13, Issue 4 (Autumn 2023)
PTJ 2023, 13(4): 253-266 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Alipour Abedi F, Saidie P, Rahmani Nia F, Saberi A. The Effect of Eight Weeks of Training Aerobic and Resistance on Selected Functional Capacities Among Women With Depression and Multiple Sclerosis. PTJ 2023; 13 (4) :253-266
URL: http://ptj.uswr.ac.ir/article-1-589-en.html
URL: http://ptj.uswr.ac.ir/article-1-589-en.html
1- Department of Exercise Physiology, Faculty of Physical Education and Sport Sciences, University of Guilan, Rasht, Iran.
2- Department of Neurology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Neurology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Keywords: Aerobic training, Resistance training, Functional capacities, Depression, Multiple sclerosis
Full-Text [PDF 535 kb]
(976 Downloads)
| Abstract (HTML) (2930 Views)
Full-Text: (1070 Views)
1. Introduction
An estimated 2.8 million people worldwide were affected by multiple sclerosis (MS) in 2020. Recognition of pediatric MS has increased dramatically, with 30,000 MS cases being diagnosed in people under the age of 18 in 47 countries. MS is the central nervous system (CNS) inflammation, specified by representative focal lesions, as well as scattered tissue alterations [1]. MS is an autoimmune illness linked to adaptive immune deregulation, directing to the periodical entrance of immune cells into the CNS and consequent tissue lesions with signs of neurologic malfunction [2]. MS etiology is not yet known [3]. The nervous system is generally damaged in MS, including the nerve fibers, the cells that produce myelin, and the myelin sheath. The mentioned permanent changes are called sclerosis and since these lesions occur in different parts, the disease is known as MS [4]. Excessive muscular fatigue, muscular weakness, spasticity, and imbalance difficulty walking, sight problems, changes in cognition, etc. are some complications of MS [4, 5]. The muscle mass and strength loss, primarily in the lower limbs, has been related to a lack of functionality, leading to physical passivity and subsequently affecting balance and gait, which are already present in patients recently diagnosed with the disease. Disturbance in walking is especially noticeable for resistance and speed [6]. These disorders are often evaluated using measures of performance, like the timed up-and-go (TUG) test and the 6-minute walk test (6MWT), which assess walking endurance and speed [7]. The risk of falling is considered one of the most debilitating symptoms, despite reducing the individual’s independence and mobility, so it immediately affects and especially reduces the quality of life of the individual [8]. Motor fatigue is a current sign mentioned by patients with MS and can considerably affect their capability to carry out everyday tasks. Many studies have observed positive results using exercise interventions on fatigue, depression, and stress in people with MS [9, 10, 11]. Due to the conditions of the coronavirus pandemic and the particular conditions of MS patients who suffer from immunodeficiency, the home-based exercise protocol was designed to ensure that the subjects are under safe conditions. Also, these patients often suffer from depression. Depressed people usually have problems, such as anxiety and lack of self-confidence, and for this reason, they usually do not want to participate in groups or sports in the community or clubs. In addition, people with MS have muscle weakness and fatigue and do not show interest in physical activity and movement. Also, few studies have drawn an analogy between the effectiveness of these two kinds of exercises on these patients and there are very few studies based on exercises at home, as the positive exercise effectiveness on patients with MS have been proven many times. Suitable bodily exercises are advised by many healthcare experts, which affect various aspects related to fitness in MS sufferers, like flexibility, muscle strength, cardiovascular fitness, cognition, and respiratory function [12]. Exercise affects markers of immune system function in the whole population of adults with MS. Overconcentration of almost all proinflammatory cytokines is associated with CNS inflammation seen in the pathogenesis of MS and can exacerbate demyelinating processes in the CNS. Therefore, it is important to regulate the balance between cytokines and reduce inflammation by exercise [13]. Aerobic exercise has a positive relationship with the increase in the subcortical gray matter structures’ volume, like the basal ganglia and hippocampus, among people with MS [14]. Low- and moderate-intensity resistance training in MS has been demonstrated to be secure. They do not accelerate the progression of the condition and are effective in maintaining aerobic capacity and muscle strength [15]. Resistance exercise, in the rehabilitation of people suffering from this disease, is known as an effective tool in the recovery of people suffering from this disease, and it affects basic processes, such as changes in muscle morphology, cytokines, and neurological adaptations [16]. Combined aerobic and resistance exercises provide more psychological benefits independent of disease status and related symptoms [17]. Thus, the current research aimed to examine the effectiveness of resistance and aerobic exercises on selected functional capacities and cognitive indicators in women with MS experiencing depression.,
2. Materials and Methods
Design
The current research was a semi-experimental, single-blinded study with a pre-test and post-test design. All stages of the study were accepted by the Ethics Committee of the Sport Sciences Research Institute (SSRI), and subsequently, the selected subjects, after being informed well about the purpose of the research and completing the training protocol, were randomly selected and completed the consent form. They were randomly placed in one out of the three resistance training, control, and aerobic groups.
Participants
The statistical population was women with MS from the Ba’ath Hospital, Guilan selected by the available sampling, but the division of the samples into groups was randomized. Thirty individuals with depression and no contraindications for exercise were included in the study. The participants had an expanded disability status scale (EDSS) score range of 0-5.5 and were aged between 25 and 50 years. The study employed a single-blind method under the monitoring of a neurologist. The subjects were divided into three 10-people groups (resistance exercises, aerobic exercises, and control). The control group did not do any exercises during the protocol and followed their inactivity lifestyle until the end of the protocol. The inclusion criteria were as follows: 1) Age of 25 to 50 years old; 2) Body mass index (BMI) equal to or bigger than 18.5 kg/m2 and less than 40 kg/m2; 3) Not taking medication without coordination; 4) Performing physical activity or physical exercises in the last six months or being inactive; 5) No recent change in diet; 6) Suffering from depression; 7) The EDSS score range of 0-5.5.
Measurement
The training was designed to be conducted at home. Six virtual briefing sessions were held, covering topics, including how to perform stretching, warming up, cooling down, and the main body of each exercise. In the resistance group, participants were also given orders on how to properly wear the vest and position the weights. Also, training videos were provided to all participants. Functional tests were taken two weeks before the start of the protocol. It should be noted that the resistance group was trained three days a week and the aerobic group two days a week, and at the end of each session, the evaluator made a phone call for both groups, and the subjects’ heart rate, fatigue intensity, and exercise intensity of the patients were recorded based on the Borg scale (RPE). The protocol entirely was performed under a neurologist, a clinical psychologist, and a PhD in sports physiology supervision. This research was conducted during the COVID-19 pandemic, taking into consideration that individuals with MS may experience weakness and disability. All exercises and answers to questionnaires were done in completely hygienic conditions at home. Two weeks before the start of the exercise protocol, the subjects recorded their menstruation time in the personal information form. The Beck depression inventory (BDI-II) [18], perceived stress scale (PSS) [19], fatigue severity scale (FSS) [20], and International physical activity questionnaire (IPAQ) [21] were provided to the subjects in a virtual form through the Porsline website at a certain time after the premenstrual syndrome (PMS). Numerous symptoms have been attributed to this syndrome, and it covers a wide spectrum that includes behavioral, emotional, and physical domains [22]. All questionnaires and functional tests were re-evaluated in the post-stage after eight weeks.
Questionnaires
IPAQ: IPAQ evaluates the intensity, duration, and frequency of bodily activity during the last week. It focuses on four areas: (1) during leisure time (2) during transportation, (3) at work, and (4) during household activities. The severity of referred diverse activities can be constituted in metabolic equivalents (METs) which makes the energy cost several times the resting energy cost. One minute of moderate home activity included three METs and eight robust severity activities. Sedentary performance is assessed as an additional area provided in minutes each week. This questionnaire was used to assess the physical activity of the subjects during a phase before the start of the protocol to ensure the immobility of the subjects [23].
PSS: The perceived stress was assessed via the 10-item PSS. By scoring a total of 10 items, ranging from 0 to 40, a total score was measured. To obtain a dichotomous variable, a threshold of 14 was utilized, a score of less than 14 was classified as “low perceived stress” and a score of 14 or more was classified as “high perceived stress” [24].
FSS: FSS is a 9-item self-report scale to evaluate fatigue, its intensity, and its effect on particular activities. The answers were classified, varying from one (strongly disagree) to seven (strongly agree) based on a 7-point scale. Higher scores on the FSS show more severe fatigue symptoms and interference with daily activities [25].
BDI-II: BDI-II is one of the most utilized self-report scales to evaluate the severity of depressive symptoms. Severity cutoff scores were originally proposed by Beck et al. in 1996 and can be defined as 0 to 13 as minimal, 14 to 19 as mild, 20 to 28 as moderate and severe, and 29 and above as depression [26].
Functional capacity
TUG: a widely accessible assessment of overall physical function, the TUG test, measures the time spent to accomplish a specific task. In the TUG, participators stand up from a chair, go around a cone 3 meters away, and turn back to the chair once again to sit down [27].
10-minute walk test (10MWT): A simple and commonly used assessment is the 10MWT where the patient is instructed to walk at their usual speed for a distance of 10 m. The outcome is the time required to finish the task [28, 29].
6-minute walk test (6MWT): a famous device, the 6MWT, is to assess stamina in the sub-maximal status and capacity to walk. The distance of 6MWT is related to health-related quality of life, maximal workload measured on a cardiopulmonary exercise test, and peak oxygen consumption [28, 30].
30-second chair stand test (30 CST): Using the 30 CST, the strength of the functional lower limb was measured. With the patient positioned on an armless chair, the test was administered.
According to the order of the examiner, the patient stood straight up and immediately sat down once more till the back reached the chair backrest. The repetition of the referred process was as often as feasible in a half minute. The examiner was upright near the patient to prevent any potential issues, like fallings but did not help the patient during the test. The total number of complete standings was noted This assessment was conducted two weeks before the commencement of the training.
Training protocol
Resistance training
Based on the periodization model, a 2-week pilot and eight-week training were carried out, with a focus on strengthening people’s mobility, balance, and strength. The periodic model contained training sessions for two weeks and exercise for eight weeks at home. This periodic technique is used by both athletes and people without a regular training history [32]. The training duration was eight weeks, which included a hypertrophy phase (four weeks) and a strength and power phase (four weeks), and three sessions were held per week. The participants engaged in a warm-up routine consisting of 5-10 minutes of movements, such as walking, slow jogging, and gentle-to-moderate-intensity stretching exercises [33, 34]. This was followed by 25-30 minutes of main exercises, and finally, 5-10 minutes of stretching movements to cool down the entire body [35]. Participants were asked to do the exercises at their own speed and relax between sets if essential. To increase the intensity of the training, in the resistance training program, weight vests were used. The training repetitions and intensity were different for each person and changed every two weeks. There was a 4-2-minute rest between each set. The initial resistance of the vest was 0.5% of each person’s body weight. According to the person’s score of EDSS and the initial results of the performance evaluation tests (TUG, 6MWT, 10MWT, 30 CST), the initial number of repetitions for each individual’s movements was determined. Movements contained leg curls, forward lunges, heel-toe raises (legs standing), step-ups, and chair raises.
The training program in the first and third weeks included exercises in two sets with repetitions ranging from 8 to 12, which the person performed with the minimum ability of eight repetitions and the maximum ability of 12 repetitions. The number of repetitions of each movement was determined according to the doctor’s prescription and the patient’s clinical symptoms.
It should be noted that in the third week, according to the patient’s ability (clinical symptoms during the last two weeks), based on the research team’s prescription, and the individual’s EDSS score, an additional weight ranging from 0.5 to 1.5 percent of their body weight was added to the weight of the vest.
In the second and fourth weeks, the number of sets increased from two to three sets (hypertrophy phase), and the repetition of each movement was modified between 8 and 12 repetitions according to the patient’s reports, clinical symptoms, and the accompanying doctor’s prescription.
The fifth to eighth weeks are the strength and power phase, where the exercises are done in two sets with 8-10 repetitions. The way to increase intensity and repetition was the same as before, according to the individual’s performance, but the maximum number of repetitions was ten [36].
Aerobic training
The type of aerobic exercise of these patients was walking (with a treadmill or outdoors), which started two days a week for 10 minutes and over time was performed for 30 minutes in eight weeks. According to their abilities, they were able to divide their walking time into five parts. The intensity of the training was between 11 and 13 of 20 or 40-60% of HRpeak (the maximum heart rate) according to the rating of perceived exertion (RPE) during all public sessions. The duration of exercise was adjusted based on the individual’s tolerance level for MS. Subjects were asked to add 5 minutes to their walking time once every two weeks, if they were compatible with the intensity and duration of the previous exercise, and take a maximum of 1-2 minutes of rest every five minutes. At the end of each session, before and immediately after the activity, the heart rate was measured in each session [37].
Statistical analyses
To assess the normality of the data distribution, the data were analyzed by the Shapiro-Wilk test, and the repeated-measure ANOVA was conducted to determine alterations both within and between groups. Additionally, the Bonferroni post hoc test was employed for further analysis. Inferential tests were performed using SPSS software, version 26, and drawing shapes were made using the Word software. A level of significance of p≤0.05 was considered.
3. Results
The results of two-way ANOVA indicated no notable dissimilarity among the three groups in height, weight, age, BMI, IPAQ score, and EDSS score (p>0.05), and for all the above factors, the three groups were homogeneous (Table 1).
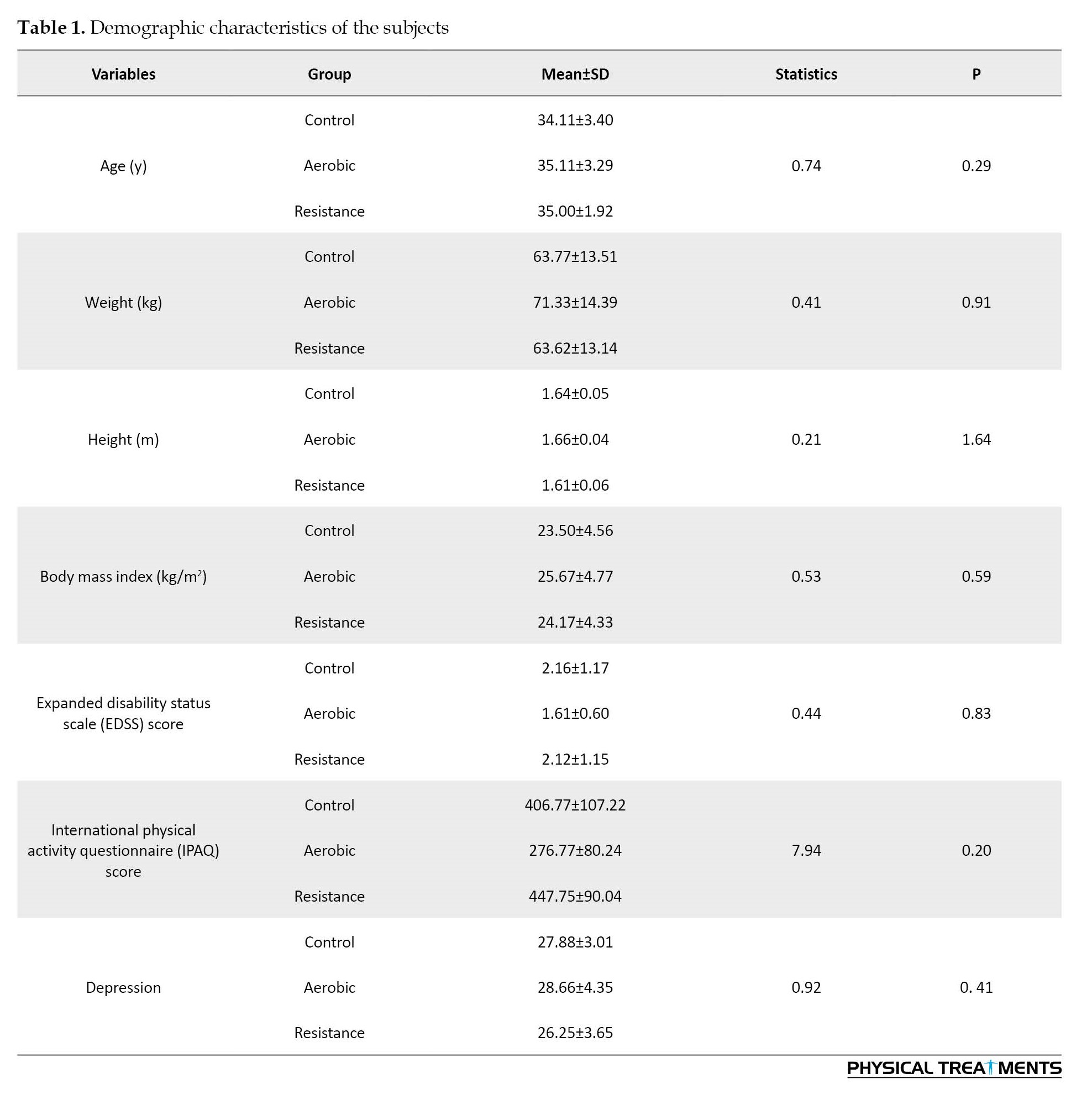
Considering the results of the two-way ANOVA, there were significant differences between the resistance, aerobic and control groups in perceived stress (p=0.01), the 6MWT (P=0.001), depression level (p=0.001), 30 CST (p=0.001), and the 10MWT (p=0.01) (Table 2).
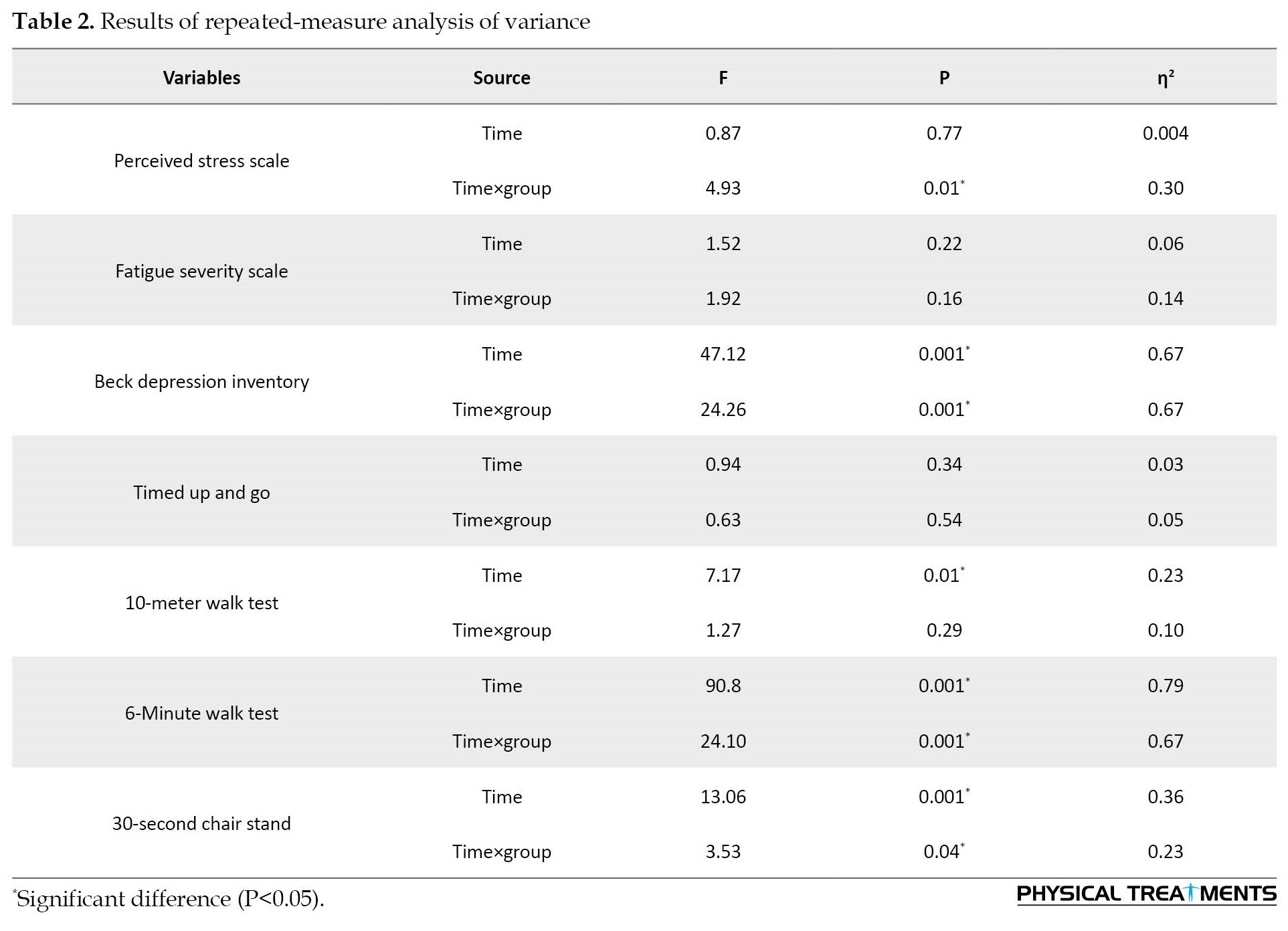
As shown in Table 3, the Bonferroni test results on within-group changes between the pre-test and post-test of each group indicated that resistance exercises had a significant effect on the perceived stress (p=0.04), 10MWT speed (p=0.01), depression level (p=0.001), 6MWT (p=0.001), and 30 CST (p=0.001) had.
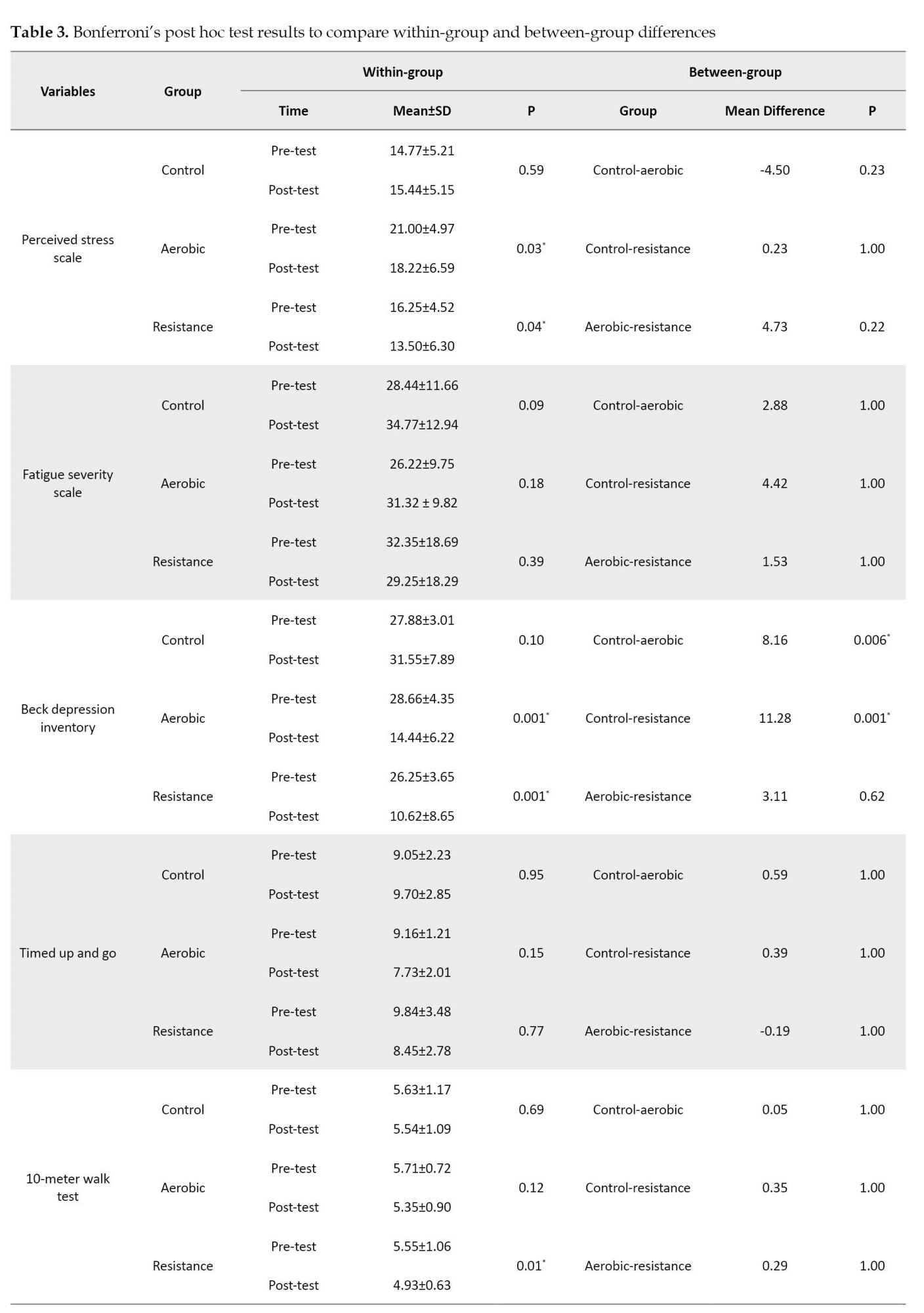
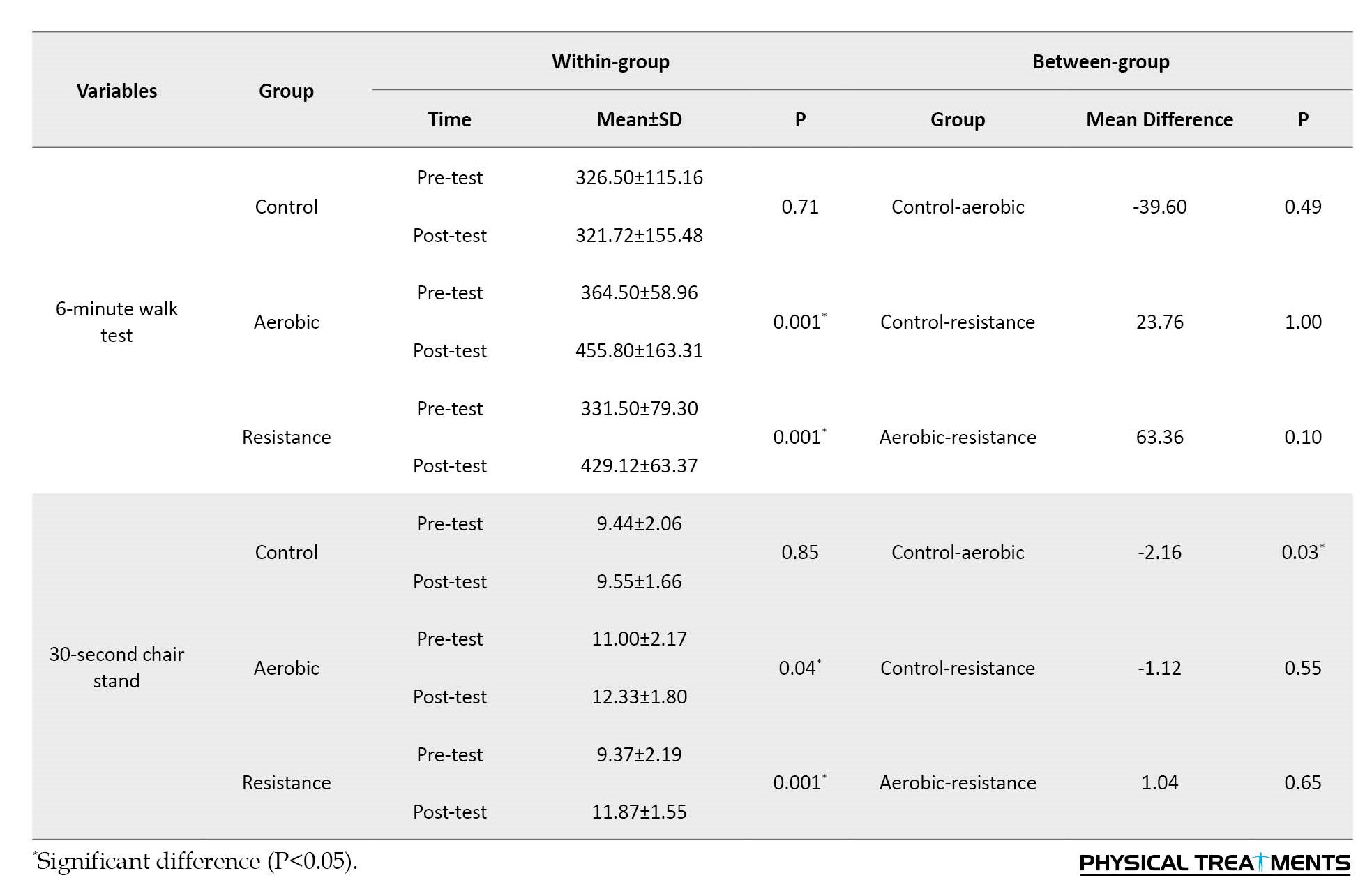
Also, aerobic exercises had a significant effect on perceived stress (p=0.03), depression level (p=0.001), 6MWT (P=0.001), and 30CST (p=0.04). The between-group results of the Bonferroni post hoc test demonstrated a significant difference between the control and resistance (p=0.001) and the control and aerobic (p=0.006) groups in the level of depression. It had a significant effect on the functional strength of the lower limbs (30 CST). However, no notable dissimilarity was seen in other variables.
4. Discussion
The aim of this research was to survey the effectiveness of eight weeks of aerobic and resistance exercises on selected cognitive and functional capacities in women with MS. The results of the current research demonstrated that aerobic and resistance training had a notable effect on depression, perceived stress, and selected functional capacities of patients with MS. However, no significant effects were found on the level of fatigue and functional balance test concerning other variables. Studies focused on home-based training for individuals with MS are relatively scarce, and there is no consensus concerning the effectiveness of training on cognitive and physical factors in this population. Also, limited studies have been conducted on the intervention effectiveness of education in some factors of the present research in this type of patient. Therefore, the current research was carried out to examine the effect of two training types on these factors in patients with MS.
In a systematic review including articles published from 2010 to the present that support the present study, exercise as a single, adjunctive, or combined treatment was shown to be effective for depression in all age groups (mainly 25-45 years) and the advantage of exercise therapy was similar to conventional depression therapy. To diminish depressive signs moderate-intensity exercise is sufficient; however, higher-dose exercise is generally an improvement in functioning. Exercise therapy has become more widely used because of its benefits for systemic functions, emotional states, and the cardiovascular system [38]. A narrative review of systematic reviews and meta-analyses on the current state of evidence for three common treatments for depression in people with MS (i.e. exercise, cognitive-behavioral therapy, and antidepressants) found that there is no gold standard for depression and single therapy and combination therapies have been suggested for depression management in MS. However, there is little evidence for the use of combination therapy for depression and its outcomes in people with MS [39].
Studies aimed at investigating the effect of exercise training on perceived stress in patients with MS are very few; thus, relatively relevant studies were examined. For example, 60 participators with MS were randomly assigned to cognition-targeted exercise (CTE) and the symptom-targeted exercise (STE). Participants in the control group, in addition to the STE program (standard physical therapy program), also performed eight 50-minute sessions of weekly cognitive-behavioral therapy (CBT). In contrast, participants in the experimental group underwent eight weekly 50-minute CBT sessions in addition to the CTE program. The hospital anxiety and depression scale (HADS) and the PSS were utilized and there was a significant difference between the groups during follow-up and post-test visits regarding all clinical outcomes [40]. In another study with inconsistent results with the current study on 47 women with relapsing-remitting multiple sclerosis (RRMS), physical activity prevented the occurrence of relapse or inactivity could lead to relapse. There were weak and inconsistent results regarding perceived stress as a mediator or moderator of the relationship between physical activity and relapse in MS [41].
The evidence of examining the frequency and severity of fatigue in 100 patients with RRMS and its relationship with general physical activity and disease-related disability showed that fatigue is a common symptom in MS patients; patients with less physical activity and more MS-related disabilities, suffer significant fatigue that negatively affects cognitive, psychosocial, and physical functioning [42]. In another study involving 68 patients with MS, two groups were investigated: an exercise intervention of 24 weeks of progressive aerobic exercise (PAE) followed by self-directed physical activity, and a wait-list control group (24 weeks of conventional lifestyle followed by PAE) The PAE group performed exercises in two sessions of 30-60 minutes per week with an intensity of 65-95% of the maximum heart rate and the 24-week waiting list control subjects had normal life followed by aerobic exercise. The aerobic exercise was not effective in the intensity of fatigue; however, significant effectiveness was seen in the 6MWT [43]. In a study conducted by Grazioli et al. on the effectiveness of a 12-week merged training intervention (aerobic and resistance exercise) on the quality of life, fatigue perception, walking ability, severity of disease, and balance in MS patients, bodily activity was effective in MS patients and supported the combined utilization of aerobic and resistance exercises to attain psychological and functional therapeutic results. Also, an increase in the ability to walk, balance, reduction of severity, fatigue, and depression of the disease was observed [44]. The results of a study including 66 people with MS performing eight weeks of intermittent exercise with a maximum of 60% to 75% W, supported exercise as an effective therapeutic intervention to improve functional parameters, depression, and fatigue apart from the beginning regardless of the baseline weight status in people with MS [45]. The effectiveness of physical activity on the fatigue of people with MS is different. In the research by Riemenschneider et al., the results of 48 weeks of aerobic training on physical and cognitive performance, including measuring aerobic fitness, 6MWT, upper limb dexterity, and fatigue did not show significant differences between the groups; however, all measures of upper extremity function and gait showed low to moderate results in supporting exercise. The general state of disability as well as cognition was not affected by exercise, but the understanding of illness and the effect of weariness decreased in both groups [46].
In a study involving 30 men and women with MS, the effect of eight weeks of home-based neurofunctional training (HBNFT) TUG, 6MWT, and 10MWT was compared with home-based resistance training (HBRT) performed in three sessions per week. The results showed only a significant effect on 6MWT, while no significant changes or differences were observed in the other factors, which is in line with the findings of the current research [47]. Therefore, additional study is guaranteed to discover the role of various factors, such as exercise type, exercise environment, different exercise intensities, gender, age range, sample size, sample characteristics, and the severity and type of the disease. Investigating these aspects is important to gain a deeper understanding of their impact on outcomes related to exercise interventions. In MS, people with more severe fatigue use extra hours during the day without physical activity. People who have more day-to-day bodily activities have better physical and neuromuscular functions. This interrelationship between body function, sedentary behavior, and fatigue draws attention to the importance of reducing the perception of fatigue in MS patients [48]. Among people with MS, there is evidence that these elements are factors in response to physical activity interventions and exercises. For example, the development of lower limb muscle strength is related to an increase in neural drive after a 12-week continuous resistance training program among people with MS [49]. Regarding primary fatigue, attention is paid to changes in the CNS under the effect of regular exercise (reduction of neurodegeneration, improvement of synaptic plasticity and neurogenesis through increased BDNF level), immunological changes (decrease of inflammation), and neuroendocrine changes via normalization of hypothalamic–pituitary–adrenal axis dysfunction [50].
Some factors are recognized to affect the cognitive dysfunction level. Orderly bodily activity, a healthy diet, an absence of addiction, and appropriate comorbidities control can have a positive effect on the cognition of MS patients [51]. Exercise can promote molecular alteration that diverts a chronic pro-inflammatory state to an anti-inflammatory state in the central and peripheral nervous systems [52]. Regarding the effectiveness of mind-body interventions, it can be attributed to the level of GABA in the thalamus, which naturally acts as an anti-anxiety agent. In addition, relevant evidence shows that meditation induces interior relief of the autonomic nervous system, without leading to lethargy, while increasing the activity of the immune system, modulating various neurotransmitters, like norepinephrine and serotonin, thereby enhancing various psychological and cognitive features containing depression [53]. Also, physical activity probably increases self-confidence and good sensation in a person, which leads to an improvement in depression. In addition, exercise may improve antioxidant defenses and neurotrophic factors, which can reduce CNS susceptibility to neurodegeneration. Exercise exposure (preconditioning) may act as a mechanism to increase stress resistance and may maintain neuronal survival under severe stress conditions. Given that cerebral atrophy and axonal loss appear early in the disease, exercise administration in the acute phase can increase neuroplasticity, neurodegeneration, and neuroprotection and reduce long-term disability [54]. People with MS are challenged to lead an active lifestyle through fitness. Although exercise prescription has been considered a therapeutic strategy to minimize the loss of functional capacity in chronic illness, it is underused as an interference strategy in the MS population.
5. Conclusion
Scientific, medical, and sports evidence has shown that MS patients suffer from physical and mental problems due to lack of movement and exercise. It is possible to reduce many complications of MS by performing sports movements and basic mobility, according to the physical conditions of each person. Home-based physical activity, including resistance and aerobic exercises, can affect some factors, including depression, perceived stress, 6MWT, 10-MWT, and 30 SCT in people with MS. Thus, sports trainers, clinical exercise physiologists, therapists, and physiotherapists are recommended to use resistance and aerobic exercises to improve the physical and mental condition of patients with MS.
Limitations
Some limitations of this study included the absence of dietary assessment, the limited sample size before and during the intervention protocol, and the lack of examination and control of stressful factors during exercise performance. It has been reported that stress management treatment can reduce inflammation and enhance neuroprotection in conditions, such as MS, which is exacerbated by stress. Therefore, future studies should consider factors, such as age, the severity of MS, exercise intensity and type, duration of the exercise protocol, gender, and geographic location of the subjects. Also, this research was carried out throughout the pandemic of COVID-19 and it is better to conduct the protocol in a more controlled environment. Depression, fatigue, and other variables should be measured by accurate tools along with sports interventions. Taking these factors into account can provide valuable insights for further research in this field.
Ethical Considerations
Compliance with ethical guidelines
The current research was approved by the Ethics Committee of Sport Sciences Research Institute of Iran (SSRI) (Code: IR.SSRI.REC.1400.1109).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the research assistant of the University of Guilan and all the subjects who sincerely participated in the present study.
References
An estimated 2.8 million people worldwide were affected by multiple sclerosis (MS) in 2020. Recognition of pediatric MS has increased dramatically, with 30,000 MS cases being diagnosed in people under the age of 18 in 47 countries. MS is the central nervous system (CNS) inflammation, specified by representative focal lesions, as well as scattered tissue alterations [1]. MS is an autoimmune illness linked to adaptive immune deregulation, directing to the periodical entrance of immune cells into the CNS and consequent tissue lesions with signs of neurologic malfunction [2]. MS etiology is not yet known [3]. The nervous system is generally damaged in MS, including the nerve fibers, the cells that produce myelin, and the myelin sheath. The mentioned permanent changes are called sclerosis and since these lesions occur in different parts, the disease is known as MS [4]. Excessive muscular fatigue, muscular weakness, spasticity, and imbalance difficulty walking, sight problems, changes in cognition, etc. are some complications of MS [4, 5]. The muscle mass and strength loss, primarily in the lower limbs, has been related to a lack of functionality, leading to physical passivity and subsequently affecting balance and gait, which are already present in patients recently diagnosed with the disease. Disturbance in walking is especially noticeable for resistance and speed [6]. These disorders are often evaluated using measures of performance, like the timed up-and-go (TUG) test and the 6-minute walk test (6MWT), which assess walking endurance and speed [7]. The risk of falling is considered one of the most debilitating symptoms, despite reducing the individual’s independence and mobility, so it immediately affects and especially reduces the quality of life of the individual [8]. Motor fatigue is a current sign mentioned by patients with MS and can considerably affect their capability to carry out everyday tasks. Many studies have observed positive results using exercise interventions on fatigue, depression, and stress in people with MS [9, 10, 11]. Due to the conditions of the coronavirus pandemic and the particular conditions of MS patients who suffer from immunodeficiency, the home-based exercise protocol was designed to ensure that the subjects are under safe conditions. Also, these patients often suffer from depression. Depressed people usually have problems, such as anxiety and lack of self-confidence, and for this reason, they usually do not want to participate in groups or sports in the community or clubs. In addition, people with MS have muscle weakness and fatigue and do not show interest in physical activity and movement. Also, few studies have drawn an analogy between the effectiveness of these two kinds of exercises on these patients and there are very few studies based on exercises at home, as the positive exercise effectiveness on patients with MS have been proven many times. Suitable bodily exercises are advised by many healthcare experts, which affect various aspects related to fitness in MS sufferers, like flexibility, muscle strength, cardiovascular fitness, cognition, and respiratory function [12]. Exercise affects markers of immune system function in the whole population of adults with MS. Overconcentration of almost all proinflammatory cytokines is associated with CNS inflammation seen in the pathogenesis of MS and can exacerbate demyelinating processes in the CNS. Therefore, it is important to regulate the balance between cytokines and reduce inflammation by exercise [13]. Aerobic exercise has a positive relationship with the increase in the subcortical gray matter structures’ volume, like the basal ganglia and hippocampus, among people with MS [14]. Low- and moderate-intensity resistance training in MS has been demonstrated to be secure. They do not accelerate the progression of the condition and are effective in maintaining aerobic capacity and muscle strength [15]. Resistance exercise, in the rehabilitation of people suffering from this disease, is known as an effective tool in the recovery of people suffering from this disease, and it affects basic processes, such as changes in muscle morphology, cytokines, and neurological adaptations [16]. Combined aerobic and resistance exercises provide more psychological benefits independent of disease status and related symptoms [17]. Thus, the current research aimed to examine the effectiveness of resistance and aerobic exercises on selected functional capacities and cognitive indicators in women with MS experiencing depression.,
2. Materials and Methods
Design
The current research was a semi-experimental, single-blinded study with a pre-test and post-test design. All stages of the study were accepted by the Ethics Committee of the Sport Sciences Research Institute (SSRI), and subsequently, the selected subjects, after being informed well about the purpose of the research and completing the training protocol, were randomly selected and completed the consent form. They were randomly placed in one out of the three resistance training, control, and aerobic groups.
Participants
The statistical population was women with MS from the Ba’ath Hospital, Guilan selected by the available sampling, but the division of the samples into groups was randomized. Thirty individuals with depression and no contraindications for exercise were included in the study. The participants had an expanded disability status scale (EDSS) score range of 0-5.5 and were aged between 25 and 50 years. The study employed a single-blind method under the monitoring of a neurologist. The subjects were divided into three 10-people groups (resistance exercises, aerobic exercises, and control). The control group did not do any exercises during the protocol and followed their inactivity lifestyle until the end of the protocol. The inclusion criteria were as follows: 1) Age of 25 to 50 years old; 2) Body mass index (BMI) equal to or bigger than 18.5 kg/m2 and less than 40 kg/m2; 3) Not taking medication without coordination; 4) Performing physical activity or physical exercises in the last six months or being inactive; 5) No recent change in diet; 6) Suffering from depression; 7) The EDSS score range of 0-5.5.
Measurement
The training was designed to be conducted at home. Six virtual briefing sessions were held, covering topics, including how to perform stretching, warming up, cooling down, and the main body of each exercise. In the resistance group, participants were also given orders on how to properly wear the vest and position the weights. Also, training videos were provided to all participants. Functional tests were taken two weeks before the start of the protocol. It should be noted that the resistance group was trained three days a week and the aerobic group two days a week, and at the end of each session, the evaluator made a phone call for both groups, and the subjects’ heart rate, fatigue intensity, and exercise intensity of the patients were recorded based on the Borg scale (RPE). The protocol entirely was performed under a neurologist, a clinical psychologist, and a PhD in sports physiology supervision. This research was conducted during the COVID-19 pandemic, taking into consideration that individuals with MS may experience weakness and disability. All exercises and answers to questionnaires were done in completely hygienic conditions at home. Two weeks before the start of the exercise protocol, the subjects recorded their menstruation time in the personal information form. The Beck depression inventory (BDI-II) [18], perceived stress scale (PSS) [19], fatigue severity scale (FSS) [20], and International physical activity questionnaire (IPAQ) [21] were provided to the subjects in a virtual form through the Porsline website at a certain time after the premenstrual syndrome (PMS). Numerous symptoms have been attributed to this syndrome, and it covers a wide spectrum that includes behavioral, emotional, and physical domains [22]. All questionnaires and functional tests were re-evaluated in the post-stage after eight weeks.
Questionnaires
IPAQ: IPAQ evaluates the intensity, duration, and frequency of bodily activity during the last week. It focuses on four areas: (1) during leisure time (2) during transportation, (3) at work, and (4) during household activities. The severity of referred diverse activities can be constituted in metabolic equivalents (METs) which makes the energy cost several times the resting energy cost. One minute of moderate home activity included three METs and eight robust severity activities. Sedentary performance is assessed as an additional area provided in minutes each week. This questionnaire was used to assess the physical activity of the subjects during a phase before the start of the protocol to ensure the immobility of the subjects [23].
PSS: The perceived stress was assessed via the 10-item PSS. By scoring a total of 10 items, ranging from 0 to 40, a total score was measured. To obtain a dichotomous variable, a threshold of 14 was utilized, a score of less than 14 was classified as “low perceived stress” and a score of 14 or more was classified as “high perceived stress” [24].
FSS: FSS is a 9-item self-report scale to evaluate fatigue, its intensity, and its effect on particular activities. The answers were classified, varying from one (strongly disagree) to seven (strongly agree) based on a 7-point scale. Higher scores on the FSS show more severe fatigue symptoms and interference with daily activities [25].
BDI-II: BDI-II is one of the most utilized self-report scales to evaluate the severity of depressive symptoms. Severity cutoff scores were originally proposed by Beck et al. in 1996 and can be defined as 0 to 13 as minimal, 14 to 19 as mild, 20 to 28 as moderate and severe, and 29 and above as depression [26].
Functional capacity
TUG: a widely accessible assessment of overall physical function, the TUG test, measures the time spent to accomplish a specific task. In the TUG, participators stand up from a chair, go around a cone 3 meters away, and turn back to the chair once again to sit down [27].
10-minute walk test (10MWT): A simple and commonly used assessment is the 10MWT where the patient is instructed to walk at their usual speed for a distance of 10 m. The outcome is the time required to finish the task [28, 29].
6-minute walk test (6MWT): a famous device, the 6MWT, is to assess stamina in the sub-maximal status and capacity to walk. The distance of 6MWT is related to health-related quality of life, maximal workload measured on a cardiopulmonary exercise test, and peak oxygen consumption [28, 30].
30-second chair stand test (30 CST): Using the 30 CST, the strength of the functional lower limb was measured. With the patient positioned on an armless chair, the test was administered.
According to the order of the examiner, the patient stood straight up and immediately sat down once more till the back reached the chair backrest. The repetition of the referred process was as often as feasible in a half minute. The examiner was upright near the patient to prevent any potential issues, like fallings but did not help the patient during the test. The total number of complete standings was noted This assessment was conducted two weeks before the commencement of the training.
Training protocol
Resistance training
Based on the periodization model, a 2-week pilot and eight-week training were carried out, with a focus on strengthening people’s mobility, balance, and strength. The periodic model contained training sessions for two weeks and exercise for eight weeks at home. This periodic technique is used by both athletes and people without a regular training history [32]. The training duration was eight weeks, which included a hypertrophy phase (four weeks) and a strength and power phase (four weeks), and three sessions were held per week. The participants engaged in a warm-up routine consisting of 5-10 minutes of movements, such as walking, slow jogging, and gentle-to-moderate-intensity stretching exercises [33, 34]. This was followed by 25-30 minutes of main exercises, and finally, 5-10 minutes of stretching movements to cool down the entire body [35]. Participants were asked to do the exercises at their own speed and relax between sets if essential. To increase the intensity of the training, in the resistance training program, weight vests were used. The training repetitions and intensity were different for each person and changed every two weeks. There was a 4-2-minute rest between each set. The initial resistance of the vest was 0.5% of each person’s body weight. According to the person’s score of EDSS and the initial results of the performance evaluation tests (TUG, 6MWT, 10MWT, 30 CST), the initial number of repetitions for each individual’s movements was determined. Movements contained leg curls, forward lunges, heel-toe raises (legs standing), step-ups, and chair raises.
The training program in the first and third weeks included exercises in two sets with repetitions ranging from 8 to 12, which the person performed with the minimum ability of eight repetitions and the maximum ability of 12 repetitions. The number of repetitions of each movement was determined according to the doctor’s prescription and the patient’s clinical symptoms.
It should be noted that in the third week, according to the patient’s ability (clinical symptoms during the last two weeks), based on the research team’s prescription, and the individual’s EDSS score, an additional weight ranging from 0.5 to 1.5 percent of their body weight was added to the weight of the vest.
In the second and fourth weeks, the number of sets increased from two to three sets (hypertrophy phase), and the repetition of each movement was modified between 8 and 12 repetitions according to the patient’s reports, clinical symptoms, and the accompanying doctor’s prescription.
The fifth to eighth weeks are the strength and power phase, where the exercises are done in two sets with 8-10 repetitions. The way to increase intensity and repetition was the same as before, according to the individual’s performance, but the maximum number of repetitions was ten [36].
Aerobic training
The type of aerobic exercise of these patients was walking (with a treadmill or outdoors), which started two days a week for 10 minutes and over time was performed for 30 minutes in eight weeks. According to their abilities, they were able to divide their walking time into five parts. The intensity of the training was between 11 and 13 of 20 or 40-60% of HRpeak (the maximum heart rate) according to the rating of perceived exertion (RPE) during all public sessions. The duration of exercise was adjusted based on the individual’s tolerance level for MS. Subjects were asked to add 5 minutes to their walking time once every two weeks, if they were compatible with the intensity and duration of the previous exercise, and take a maximum of 1-2 minutes of rest every five minutes. At the end of each session, before and immediately after the activity, the heart rate was measured in each session [37].
Statistical analyses
To assess the normality of the data distribution, the data were analyzed by the Shapiro-Wilk test, and the repeated-measure ANOVA was conducted to determine alterations both within and between groups. Additionally, the Bonferroni post hoc test was employed for further analysis. Inferential tests were performed using SPSS software, version 26, and drawing shapes were made using the Word software. A level of significance of p≤0.05 was considered.
3. Results
The results of two-way ANOVA indicated no notable dissimilarity among the three groups in height, weight, age, BMI, IPAQ score, and EDSS score (p>0.05), and for all the above factors, the three groups were homogeneous (Table 1).

Considering the results of the two-way ANOVA, there were significant differences between the resistance, aerobic and control groups in perceived stress (p=0.01), the 6MWT (P=0.001), depression level (p=0.001), 30 CST (p=0.001), and the 10MWT (p=0.01) (Table 2).

As shown in Table 3, the Bonferroni test results on within-group changes between the pre-test and post-test of each group indicated that resistance exercises had a significant effect on the perceived stress (p=0.04), 10MWT speed (p=0.01), depression level (p=0.001), 6MWT (p=0.001), and 30 CST (p=0.001) had.


Also, aerobic exercises had a significant effect on perceived stress (p=0.03), depression level (p=0.001), 6MWT (P=0.001), and 30CST (p=0.04). The between-group results of the Bonferroni post hoc test demonstrated a significant difference between the control and resistance (p=0.001) and the control and aerobic (p=0.006) groups in the level of depression. It had a significant effect on the functional strength of the lower limbs (30 CST). However, no notable dissimilarity was seen in other variables.
4. Discussion
The aim of this research was to survey the effectiveness of eight weeks of aerobic and resistance exercises on selected cognitive and functional capacities in women with MS. The results of the current research demonstrated that aerobic and resistance training had a notable effect on depression, perceived stress, and selected functional capacities of patients with MS. However, no significant effects were found on the level of fatigue and functional balance test concerning other variables. Studies focused on home-based training for individuals with MS are relatively scarce, and there is no consensus concerning the effectiveness of training on cognitive and physical factors in this population. Also, limited studies have been conducted on the intervention effectiveness of education in some factors of the present research in this type of patient. Therefore, the current research was carried out to examine the effect of two training types on these factors in patients with MS.
In a systematic review including articles published from 2010 to the present that support the present study, exercise as a single, adjunctive, or combined treatment was shown to be effective for depression in all age groups (mainly 25-45 years) and the advantage of exercise therapy was similar to conventional depression therapy. To diminish depressive signs moderate-intensity exercise is sufficient; however, higher-dose exercise is generally an improvement in functioning. Exercise therapy has become more widely used because of its benefits for systemic functions, emotional states, and the cardiovascular system [38]. A narrative review of systematic reviews and meta-analyses on the current state of evidence for three common treatments for depression in people with MS (i.e. exercise, cognitive-behavioral therapy, and antidepressants) found that there is no gold standard for depression and single therapy and combination therapies have been suggested for depression management in MS. However, there is little evidence for the use of combination therapy for depression and its outcomes in people with MS [39].
Studies aimed at investigating the effect of exercise training on perceived stress in patients with MS are very few; thus, relatively relevant studies were examined. For example, 60 participators with MS were randomly assigned to cognition-targeted exercise (CTE) and the symptom-targeted exercise (STE). Participants in the control group, in addition to the STE program (standard physical therapy program), also performed eight 50-minute sessions of weekly cognitive-behavioral therapy (CBT). In contrast, participants in the experimental group underwent eight weekly 50-minute CBT sessions in addition to the CTE program. The hospital anxiety and depression scale (HADS) and the PSS were utilized and there was a significant difference between the groups during follow-up and post-test visits regarding all clinical outcomes [40]. In another study with inconsistent results with the current study on 47 women with relapsing-remitting multiple sclerosis (RRMS), physical activity prevented the occurrence of relapse or inactivity could lead to relapse. There were weak and inconsistent results regarding perceived stress as a mediator or moderator of the relationship between physical activity and relapse in MS [41].
The evidence of examining the frequency and severity of fatigue in 100 patients with RRMS and its relationship with general physical activity and disease-related disability showed that fatigue is a common symptom in MS patients; patients with less physical activity and more MS-related disabilities, suffer significant fatigue that negatively affects cognitive, psychosocial, and physical functioning [42]. In another study involving 68 patients with MS, two groups were investigated: an exercise intervention of 24 weeks of progressive aerobic exercise (PAE) followed by self-directed physical activity, and a wait-list control group (24 weeks of conventional lifestyle followed by PAE) The PAE group performed exercises in two sessions of 30-60 minutes per week with an intensity of 65-95% of the maximum heart rate and the 24-week waiting list control subjects had normal life followed by aerobic exercise. The aerobic exercise was not effective in the intensity of fatigue; however, significant effectiveness was seen in the 6MWT [43]. In a study conducted by Grazioli et al. on the effectiveness of a 12-week merged training intervention (aerobic and resistance exercise) on the quality of life, fatigue perception, walking ability, severity of disease, and balance in MS patients, bodily activity was effective in MS patients and supported the combined utilization of aerobic and resistance exercises to attain psychological and functional therapeutic results. Also, an increase in the ability to walk, balance, reduction of severity, fatigue, and depression of the disease was observed [44]. The results of a study including 66 people with MS performing eight weeks of intermittent exercise with a maximum of 60% to 75% W, supported exercise as an effective therapeutic intervention to improve functional parameters, depression, and fatigue apart from the beginning regardless of the baseline weight status in people with MS [45]. The effectiveness of physical activity on the fatigue of people with MS is different. In the research by Riemenschneider et al., the results of 48 weeks of aerobic training on physical and cognitive performance, including measuring aerobic fitness, 6MWT, upper limb dexterity, and fatigue did not show significant differences between the groups; however, all measures of upper extremity function and gait showed low to moderate results in supporting exercise. The general state of disability as well as cognition was not affected by exercise, but the understanding of illness and the effect of weariness decreased in both groups [46].
In a study involving 30 men and women with MS, the effect of eight weeks of home-based neurofunctional training (HBNFT) TUG, 6MWT, and 10MWT was compared with home-based resistance training (HBRT) performed in three sessions per week. The results showed only a significant effect on 6MWT, while no significant changes or differences were observed in the other factors, which is in line with the findings of the current research [47]. Therefore, additional study is guaranteed to discover the role of various factors, such as exercise type, exercise environment, different exercise intensities, gender, age range, sample size, sample characteristics, and the severity and type of the disease. Investigating these aspects is important to gain a deeper understanding of their impact on outcomes related to exercise interventions. In MS, people with more severe fatigue use extra hours during the day without physical activity. People who have more day-to-day bodily activities have better physical and neuromuscular functions. This interrelationship between body function, sedentary behavior, and fatigue draws attention to the importance of reducing the perception of fatigue in MS patients [48]. Among people with MS, there is evidence that these elements are factors in response to physical activity interventions and exercises. For example, the development of lower limb muscle strength is related to an increase in neural drive after a 12-week continuous resistance training program among people with MS [49]. Regarding primary fatigue, attention is paid to changes in the CNS under the effect of regular exercise (reduction of neurodegeneration, improvement of synaptic plasticity and neurogenesis through increased BDNF level), immunological changes (decrease of inflammation), and neuroendocrine changes via normalization of hypothalamic–pituitary–adrenal axis dysfunction [50].
Some factors are recognized to affect the cognitive dysfunction level. Orderly bodily activity, a healthy diet, an absence of addiction, and appropriate comorbidities control can have a positive effect on the cognition of MS patients [51]. Exercise can promote molecular alteration that diverts a chronic pro-inflammatory state to an anti-inflammatory state in the central and peripheral nervous systems [52]. Regarding the effectiveness of mind-body interventions, it can be attributed to the level of GABA in the thalamus, which naturally acts as an anti-anxiety agent. In addition, relevant evidence shows that meditation induces interior relief of the autonomic nervous system, without leading to lethargy, while increasing the activity of the immune system, modulating various neurotransmitters, like norepinephrine and serotonin, thereby enhancing various psychological and cognitive features containing depression [53]. Also, physical activity probably increases self-confidence and good sensation in a person, which leads to an improvement in depression. In addition, exercise may improve antioxidant defenses and neurotrophic factors, which can reduce CNS susceptibility to neurodegeneration. Exercise exposure (preconditioning) may act as a mechanism to increase stress resistance and may maintain neuronal survival under severe stress conditions. Given that cerebral atrophy and axonal loss appear early in the disease, exercise administration in the acute phase can increase neuroplasticity, neurodegeneration, and neuroprotection and reduce long-term disability [54]. People with MS are challenged to lead an active lifestyle through fitness. Although exercise prescription has been considered a therapeutic strategy to minimize the loss of functional capacity in chronic illness, it is underused as an interference strategy in the MS population.
5. Conclusion
Scientific, medical, and sports evidence has shown that MS patients suffer from physical and mental problems due to lack of movement and exercise. It is possible to reduce many complications of MS by performing sports movements and basic mobility, according to the physical conditions of each person. Home-based physical activity, including resistance and aerobic exercises, can affect some factors, including depression, perceived stress, 6MWT, 10-MWT, and 30 SCT in people with MS. Thus, sports trainers, clinical exercise physiologists, therapists, and physiotherapists are recommended to use resistance and aerobic exercises to improve the physical and mental condition of patients with MS.
Limitations
Some limitations of this study included the absence of dietary assessment, the limited sample size before and during the intervention protocol, and the lack of examination and control of stressful factors during exercise performance. It has been reported that stress management treatment can reduce inflammation and enhance neuroprotection in conditions, such as MS, which is exacerbated by stress. Therefore, future studies should consider factors, such as age, the severity of MS, exercise intensity and type, duration of the exercise protocol, gender, and geographic location of the subjects. Also, this research was carried out throughout the pandemic of COVID-19 and it is better to conduct the protocol in a more controlled environment. Depression, fatigue, and other variables should be measured by accurate tools along with sports interventions. Taking these factors into account can provide valuable insights for further research in this field.
Ethical Considerations
Compliance with ethical guidelines
The current research was approved by the Ethics Committee of Sport Sciences Research Institute of Iran (SSRI) (Code: IR.SSRI.REC.1400.1109).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the research assistant of the University of Guilan and all the subjects who sincerely participated in the present study.
References
- Stock B, Shrestha M, Seiler A, Foerch C, Hattingen E, Steinmetz H, et al. Distribution of cortical diffusion tensor imaging changes in multiple sclerosis. Frontiers in Physiology. 2020; 11:116. [DOI:10.3389/fphys.2020.00116] [PMID]
- Carlström KE, Ewing E, Granqvist M, Gyllenberg A, Aeinehband S, Enoksson SL, et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nature Communications. 2019; 10(1):3081. [DOI:10.1038/s41467-019-11139-3] [PMID]
- Perriot S, Mathias A, Perriard G, Canales M, Jonkmans N, Merienne N, et al. Human induced pluripotent stem cell-derived astrocytes are differentially activated by multiple sclerosis-associated cytokines. Stem Cell Reports. 2018; 11(5):1199-210. [DOI:10.1016/j.stemcr.2018.09.015] [PMID]
- Shoeibi A, Khodatars M, Jafari M, Moridian P, Rezaei M, Alizadehsani R, et al. Applications of deep learning techniques for automated multiple sclerosis detection using magnetic resonance imaging: A review. Computers in Biology and Medicine. 2021; 136:104697. [DOI:10.1016/j.compbiomed.2021.104697] [PMID]
- Cakt BD, Nacir B, Genç H, Saraçoğlu M, Karagöz A, Erdem HR, et al. Cycling progressive resistance training for people with multiple sclerosis: A randomized controlled study. American Journal of Physical Medicine & Rehabilitation. 2010; 89(6):446-57. [DOI:10.1097/PHM.0b013e3181d3e71f] [PMID]
- Cuerda-Ballester M, Proaño B, Alarcón-Jimenez J, de Bernardo N, Villaron-Casales C, Lajara Romance JM, et al. Improvements in gait and balance in patients with multiple sclerosis after treatment with coconut oil and epigallocatechin gallate. A pilot study. Food & Function. 2023; 14(2):1062-71. [DOI:10.1039/D2FO02207A] [PMID]
- Marotta N, de Sire A, Marinaro C, Moggio L, Inzitari MT, Russo I, et al. Efficacy of Transcranial Direct Current Stimulation (tDCS) on balance and gait in multiple sclerosis patients: A machine learning approach. Journal of Clinical Medicine. 2022; 11(12):3505. [DOI:10.3390/jcm11123505] [PMID]
- Castellano-Aguilera A, Biviá-Roig G, Cuenca-Martínez F, Suso-Martí L, Calatayud J, Blanco-Díaz M, Casaña J. Effectiveness of virtual reality on balance and risk of falls in people with multiple sclerosis: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):14192. [DOI:10.3390/ijerph192114192] [PMID]
- Leodori G, Mancuso M, Maccarrone D, Tartaglia M, Ianniello A, Certo F, et al. Neural bases of motor fatigue in multiple sclerosis: A multimodal approach using neuromuscular assessment and TMS-EEG. Neurobiology of Disease. 2023; 180:106073. [DOI:10.1016/j.nbd.2023.106073] [PMID]
- Torres-Costoso A, Martínez-Vizcaíno V, Reina-Gutiérrez S, Álvarez-Bueno C, Guzmán-Pavón MJ, Pozuelo-Carrascosa DP, et al. Effect of exercise on fatigue in multiple sclerosis: A network meta-analysis comparing different types of exercise. Archives of Physical Medicine and Rehabilitation. 2022; 103(5):970-87. e18. [DOI:10.1016/j.apmr.2021.08.008] [PMID]
- Kneebone II, Van Zanden BE, Dorstyn DS, Roberts RM, Lord SR, Querstret D, et al. Relaxation and related therapies for people with multiple sclerosis (MS): A systematic review. Clinical Rehabilitation. 2022; 36(7):883-99. [DOI:10.1177/02692155221091509] [PMID]
- Amin NS, El Tayebi HM. More gain, less pain: How resistance training affects immune system functioning in multiple sclerosis patients: A review. Multiple Sclerosis and Related Disorders. 2023; 69:104401. [DOI:10.1016/j.msard.2022.104401] [PMID]
- Negaresh R, Motl RW, Mokhtarzade M, Dalgas U, Patel D, Shamsi MM, et al. Effects of exercise training on cytokines and adipokines in multiple sclerosis: A systematic review. Multiple Sclerosis and Related Disorders. 2018; 24:91-100. [DOI:10.1016/j.msard.2018.06.008] [PMID]
- Motl RW, Sandroff BM, DeLuca J. Exercise training and cognitive rehabilitation: A symbiotic approach for rehabilitating walking and cognitive functions in multiple sclerosis? Neurorehabilitation and Neural Repair. 2016; 30(6):499-511. [DOI:10.1177/1545968315606993] [PMID]
- Derikx TCA, Brands IMH, Goedhart AT, Hoens WH, Heijenbrok-Kal MH, Van Den Berg-Emons RHJG. High-Volume and high-intensity functional training in patients with multiple sclerosis: A pilot study on feasibility and functional capacity. Journal of Rehabilitation Medicine-Clinical Communications. 2022; 5:2047. [DOI:10.2340/jrmcc.v5.2047] [PMID]
- Kjølhede T, Vissing K, Dalgas U. Multiple sclerosis and progressive resistance training: A systematic review. Multiple Sclerosis Journal. 2012; 18(9):1215-28. [DOI:10.1177/1352458512437418] [PMID]
- Jaggers JR, Hand GA, Dudgeon WD, Burgess S, Phillips KD, Durstine JL, et al. Aerobic and resistance training improves mood state among adults living with HIV. International Journal of Sports Medicine. 2015; 36(2):175-81.[DOI:10.1055/s-0034-1385878] [PMID]
- von Glischinski M, von Brachel R, Hirschfeld G. How depressed is “depressed”? A systematic review and diagnostic meta-analysis of optimal cut points for the Beck Depression Inventory revised (BDI-II). Quality of Life Research. 2019; 28(5):1111-8. [DOI:10.1007/s11136-018-2050-x] [PMID]
- Bastianon CD, Klein EM, Tibubos AN, Brähler E, Beutel ME, Petrowski K. Perceived Stress Scale (PSS-10) psychometric properties in migrants and native Germans. BMC Psychiatry. 2020; 20(1):450. [DOI:10.1186/s12888-020-02851-2] [PMID]
- Feng C, He Q, Wu Y, Hu X, Wu J, He X, Zhao S. Psychometric properties of fatigue severity scale in Chinese systemic lupus erythematosus patients. Health and Quality of Life Outcomes. 2019; 17(1):71. [DOI:10.1186/s12955-019-1141-x] [PMID]
- Moss SJ, Czyz SH. Level of agreement between physical activity levels measured by ActiHeart and the International Physical Activity Questionnaire in persons with intellectual disability. Disability and Rehabilitation. 2018; 40(3):360-6. [DOI:10.1080/09638288.2016.1258092] [PMID]
- Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: Definitions and diagnosis. Psychoneuroendocrinology. 2003; 28 (Suppl 3):25-37. [DOI:10.1016/S0306-4530(03)00099-4] [PMID]
- Dietz de Loos A, Jiskoot G, van den Berg-Emons R, Louwers Y, Beerthuizen A, van Busschbach J, et al. The effect of tailored Short Message Service (SMS) on physical activity: Results from a three-component randomized controlled lifestyle intervention in women with PCOS. Journal of Clinical Medicine. 2023; 12(7):2466. [DOI:10.3390/jcm12072466] [PMID]
- Clendennen SL, Chen B, Sumbe A, Harrell MB. Patterns in mental health symptomatology and cigarette, E-cigarette, and marijuana use among Texas youth and young adults amid the Coronavirus Disease 2019 Pandemic. Nicotine and Tobacco Research. 2023; 25(2):266-273. [DOI:10.1093/ntr/ntac205] [PMID]
- Almegbas NR, Almutairi GR, Alosaimi RM, Alqahtani MA, Batook SG, Alfageh IA, et al. Fatigue and cognitive decline associated with depressive symptoms among community-dwelling adults.Inquiry. 2023; 60:469580231153524.[DOI:10.1177/00469580231153524] [PMID]
- Almeida S, Camacho M, Barahona-Corrêa JB, Oliveira J, Lemos R, da Silva DR, et al. Criterion and construct validity of the Beck Depression Inventory (BDI-II) to measure depression in patients with cancer: The contribution of somatic items. International Journal of Clinical and Health Psychology. 2023; 23(2):100350. [DOI:10.1016/j.ijchp.2022.100350] [PMID]
- Rössler R, Rommers N, Kim EK, Iendra L, Sofios A, Giannouli E, et al. Timed up-and-go performance is associated with objectively measured life space in patients 3 months after ischemic stroke: A cross-sectional observational study. Journal of Neurology. 2023; 270(4):1999-2009. [DOI:10.1007/s00415-022-11524-x] [PMID]
- Dong K, Meng S, Guo Z, Zhang R, Xu P, Yuan E, et al. The effects of transcranial direct current stimulation on balance and gait in stroke patients: A systematic review and meta-analysis. Frontiers in Neurology. 2021; 12:650925. [DOI:10.3389/fneur.2021.650925] [PMID]
- Sundström N, Rydja J, Virhammar J, Kollén L, Lundin F, Tullberg M. The timed up and go test in idiopathic normal pressure hydrocephalus: A Nationwide Study of 1300 patients. Fluids and Barriers of the CNS. 2022; 19(1):4.[DOI:10.1186/s12987-021-00298-5] [PMID]
- Ferté JB, Boyer FC, Taiar R, Pineau C, Barbe C, Rapin A. Impact of resistance training on the 6-minute walk test in individuals with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Annals of Physical and Rehabilitation Medicine. 2022; 65(3):101582. [DOI:10.1016/j.rehab.2021.101582] [PMID]
- Rydja J, Kollén L, Ulander M, Tullberg M, Lundin F. Physical capacity and activity in patients with idiopathic normal pressure hydrocephalus. Frontiers in Neurology. 2022; 13:845976. [DOI:10.3389/fneur.2022.845976] [PMID]
- Fleck SJ, Kraemer W. Designing resistance training programs, 4E. Champaign: Human Kinetics; 2014. [Link]
- Dehkordi AH. Influence of yoga and aerobics exercise on fatigue, pain and psychosocial status in patients with multiple sclerosis: A randomized trial. The Journal of Sports Medicine and Physical Fitness. 2016; 56(11):1417-22. [PMID]
- Sá MJ. Exercise therapy and multiple sclerosis: A systematic review. Journal of Neurology. 2014; 261(9):1651-61.[DOI:10.1007/s00415-013-7183-9] [PMID]
- White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Medicine. 2004; 34(15):1077-100. [DOI:10.2165/00007256-200434150-00005] [PMID]
- DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2004; 85(2):290-7. [DOI:10.1016/j.apmr.2003.06.003] [PMID]
- Kim Y, Lai B, Mehta T, Thirumalai M, Padalabalanarayanan S, Rimmer JH, Motl RW. Exercise training guidelines for multiple sclerosis, stroke, and Parkinson’s disease: Rapid review and synthesis. American Journal of Physical Medicine & Rehabilitation. 2019; 98(7):613-21. [DOI:10.1097/PHM.0000000000001174] [PMID]
- Xie Y, Wu Z, Sun L, Zhou L, Wang G, Xiao L, et al. The effects and mechanisms of exercise on the treatment of depression. Frontiers in Psychiatry. 2021; 12:705559. [DOI:10.3389/fpsyt.2021.705559] [PMID]
- Jones CD, Motl R, Sandroff BM. Depression in multiple sclerosis: Is one approach for its management enough? Multiple Sclerosis and Related Disorders. 2021; 51:102904. [DOI:10.1016/j.msard.2021.102904] [PMID]
- Alketbi A, Basit S, Hamza N, Walton LM, Moustafa IM. The added value of cognition-targeted exercise versus symptom-targeted exercise for multiple sclerosis fatigue: A randomized controlled pilot trial. PLoS One. 2021; 16(11):e0258752. [DOI:10.1371/journal.pone.0258752] [PMID]
- Weikert ML. Associations among physical activity, perception of stress, and relapse occurrence in multiple sclerosis.[MSc thesis]. Champaign: University of Illinois at Urbana-Champaign; 2011. [Link]
- Rzepka M, Toś M, Boroń M, Gibas K, Krzystanek E. Relationship between fatigue and physical activity in a polish cohort of multiple sclerosis patients. Medicina. 2020; 56(12):726. [DOI:10.3390/medicina56120726] [PMID]
- Langeskov-Christensen M, Hvid LG, Jensen HB, Nielsen HH, Petersen T, Stenager E, et al., Efficacy of high‐intensity aerobic exercise on common multiple sclerosis symptoms. Acta Neurologica Scandinavica. 2022; 145(2):229-38. [DOI:10.1111/ane.13540] [PMID]
- Grazioli E, Tranchita E, Borriello G, Cerulli C, Minganti C, Parisi A. The effects of concurrent resistance and aerobic exercise training on functional status in patients with multiple sclerosis. Current Sports Medicine Reports. 2019; 18(12):452-7. [DOI:10.1249/JSR.0000000000000661] [PMID]
- Negaresh R, Motl R, Mokhtarzade M, Ranjbar R, Majdinasab N, Khodadoost M, et al. Effect of short-term interval exercise training on fatigue, depression, and fitness in normal weight vs. overweight person with multiple sclerosis. Explore. 2019; 15(2):134-41. [DOI:10.1016/j.explore.2018.07.007] [PMID]
- Riemenschneider M, Hvid LG, Petersen T, Stenager E, Dalgas U. Exercise therapy in early multiple sclerosis improves physical function but not cognition: Secondary analyses from a randomized controlled trial. Neurorehabilitation and Neural Repair. 2023; 37(5):288-297.[DOI:10.1177/15459683231159659] [PMID]
- Mardaniyan Ghahfarrokhi M, Banitalebi E, Faramarzi M, Motl R. Feasibility and efficacy of home-based neurofunctional exercise vs. resistance exercise programs for ambulatory disability of multiple sclerosis patients with cognitive impairment. Multiple Sclerosis and Related Disorders. 2022; 58:103400. [DOI:10.1016/j.msard.2021.103400] [PMID]
- Andreu-Caravaca L, Ramos-Campo DJ, Chung LH, Rubio-Arias JÁ. Dosage and effectiveness of aerobic training on cardiorespiratory fitness, functional capacity, balance, and fatigue in people with multiple sclerosis: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation. 2021; 102(9):1826-39. [DOI:10.1016/j.apmr.2021.01.078] [PMID]
- Baird JF, Motl RW. Response heterogeneity with exercise training and physical activity interventions among persons with multiple sclerosis. Neurorehabilitation and Neural Repair. 2019; 33(1):3-14. [DOI:10.1177/1545968318818904] [PMID]
- Zielińska-Nowak E, Włodarczyk L, Kostka J, Miller E. New strategies for rehabilitation and pharmacological treatment of fatigue syndrome in multiple sclerosis. Journal of Clinical Medicine. 2020; 9(11):3592. [DOI:10.3390/jcm9113592] [PMID]
- Oset M, Stasiolek M, Matysiak M. Cognitive dysfunction in the early stages of multiple sclerosis-how much and how important? Current Neurology and Neuroscience Reports. 2020; 20(7):22. [DOI:10.1007/s11910-020-01045-3] [PMID]
- Ignácio ZM, da Silva RS, Plissari ME, Quevedo J, Réus GZ. Physical exercise and neuroinflammation in major depressive disorder. Molecular Neurobiology. 2019; 56(12):8323-35. [DOI:10.1007/s12035-019-01670-1] [PMID]
- Ruiz-Ariza B, Hita-Contreras F, Rodríguez-López C, Rivas-Campo Y, Aibar-Almazán A, Carcelén-Fraile MDC, et al. Effects of mind-body training as a mental health therapy in adults with diabetes mellitus type II: A systematic review. Journal of Clinical Medicine. 2023; 12(3):853. [DOI:10.3390/jcm12030853] [PMID]
- White LJ, Castellano V. Exercise and brain health-implications for multiple sclerosis: Part 1-neuronal growth factors. Sports Medicine. 2008; 38(2):91-100. [DOI:10.2165/00007256-200838030-00001] [PMID]
Type of Study: Research |
Subject:
Sport injury and corrective exercises
Received: 2023/07/21 | Accepted: 2023/08/19 | Published: 2023/10/14
Received: 2023/07/21 | Accepted: 2023/08/19 | Published: 2023/10/14
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






